DNA interstrand crosslink repair and cancer
- PMID: 21701511
- PMCID: PMC3560328
- DOI: 10.1038/nrc3088
DNA interstrand crosslink repair and cancer
Abstract
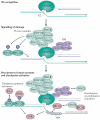
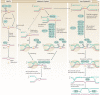
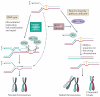
Interstrand crosslinks (ICLs) are highly toxic DNA lesions that prevent transcription and replication by inhibiting DNA strand separation. Agents that induce ICLs were one of the earliest, and are still the most widely used, forms of chemotherapeutic drug. Only recently, however, have we begun to understand how cells repair these lesions. Important insights have come from studies of individuals with Fanconi anaemia (FA), a rare genetic disorder that leads to ICL sensitivity. Understanding how the FA pathway links nucleases, helicases and other DNA-processing enzymes should lead to more targeted uses of ICL-inducing agents in cancer treatment and could provide novel insights into drug resistance.
Figures

Similar articles
-
DNA interstrand cross-link repair: understanding role of Fanconi anemia pathway and therapeutic implications.Eur J Haematol. 2013 Nov;91(5):381-93. doi: 10.1111/ejh.12169. Epub 2013 Aug 17. Eur J Haematol. 2013. PMID: 23859405 Review.
-
Fanconi anemia-independent DNA inter-strand crosslink repair in eukaryotes.Prog Biophys Mol Biol. 2020 Dec;158:33-46. doi: 10.1016/j.pbiomolbio.2020.08.005. Epub 2020 Aug 30. Prog Biophys Mol Biol. 2020. PMID: 32877700 Free PMC article. Review.
-
The FANC/BRCA Pathway Releases Replication Blockades by Eliminating DNA Interstrand Cross-Links.Genes (Basel). 2020 May 25;11(5):585. doi: 10.3390/genes11050585. Genes (Basel). 2020. PMID: 32466131 Free PMC article. Review.
-
The Fanconi anemia pathway in replication stress and DNA crosslink repair.Cell Mol Life Sci. 2012 Dec;69(23):3963-74. doi: 10.1007/s00018-012-1051-0. Epub 2012 Jun 29. Cell Mol Life Sci. 2012. PMID: 22744751 Free PMC article. Review.
-
Double-strand breaks induce homologous recombinational repair of interstrand cross-links via cooperation of MSH2, ERCC1-XPF, REV3, and the Fanconi anemia pathway.DNA Repair (Amst). 2007 Nov;6(11):1670-8. doi: 10.1016/j.dnarep.2007.06.002. Epub 2007 Jul 31. DNA Repair (Amst). 2007. PMID: 17669695 Free PMC article.
Cited by
-
5-Aza-2'-deoxycytidine causes replication lesions that require Fanconi anemia-dependent homologous recombination for repair.Nucleic Acids Res. 2013 Jun;41(11):5827-36. doi: 10.1093/nar/gkt270. Epub 2013 Apr 22. Nucleic Acids Res. 2013. PMID: 23609537 Free PMC article.
-
Formation Mechanism of Inter-Crosslink in DNA by Nitrogen Oxides Pollutants through A Diazonium Intermediate.Int J Mol Sci. 2022 Sep 13;23(18):10621. doi: 10.3390/ijms231810621. Int J Mol Sci. 2022. PMID: 36142522 Free PMC article.
-
Control of Solid-Supported Intra- vs Interstrand Stille Coupling Reactions for Synthesis of DNA-Oligophenylene Conjugates.Bioconjug Chem. 2024 Aug 21;35(8):1166-1171. doi: 10.1021/acs.bioconjchem.4c00310. Epub 2024 Jul 24. Bioconjug Chem. 2024. PMID: 39046902 Free PMC article.
-
FBW7 regulates DNA interstrand cross-link repair by modulating FAAP20 degradation.Oncotarget. 2016 Jun 14;7(24):35724-35740. doi: 10.18632/oncotarget.9595. Oncotarget. 2016. PMID: 27232758 Free PMC article.
-
NCAPH Stabilizes GEN1 in Chromatin to Resolve Ultra-Fine DNA Bridges and Maintain Chromosome Stability.Mol Cells. 2022 Nov 30;45(11):792-805. doi: 10.14348/molcells.2022.0048. Mol Cells. 2022. PMID: 36380731 Free PMC article.
References
-
- Goodman LS, et al. Nitrogen mustard therapy. Use of methyl-bis(β-chloroethyl)amine hydrochloride and tris(β-chloroethyl)amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946;132:126–132. - PubMed
-
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986;25:3912–3915. - PubMed
-
- Gargiulo D, Kumar GS, Musser SS, Tomasz M. Structural and function modification of DNA by mitomycin C. Mechanism of the DNA sequence specificity of mitomycins. Nucleic Acids Symp. Ser. 1995:169–170. - PubMed
-
- Kozekov ID, et al. DNA interchain cross-links formed by acrolein and crotonaldehyde. J. Am. Chem. Soc. 2003;125:50–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

