A ketogenic diet suppresses seizures in mice through adenosine A₁ receptors
- PMID: 21701065
- PMCID: PMC3223846
- DOI: 10.1172/JCI57813
A ketogenic diet suppresses seizures in mice through adenosine A₁ receptors
Abstract
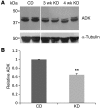
A ketogenic diet (KD) is a high-fat, low-carbohydrate metabolic regimen; its effectiveness in the treatment of refractory epilepsy suggests that the mechanisms underlying its anticonvulsive effects differ from those targeted by conventional antiepileptic drugs. Recently, KD and analogous metabolic strategies have shown therapeutic promise in other neurologic disorders, such as reducing brain injury, pain, and inflammation. Here, we have shown that KD can reduce seizures in mice by increasing activation of adenosine A1 receptors (A1Rs). When transgenic mice with spontaneous seizures caused by deficiency in adenosine metabolism or signaling were fed KD, seizures were nearly abolished if mice had intact A1Rs, were reduced if mice expressed reduced A1Rs, and were unaltered if mice lacked A1Rs. Seizures were restored by injecting either glucose (metabolic reversal) or an A1R antagonist (pharmacologic reversal). Western blot analysis demonstrated that the KD reduced adenosine kinase, the major adenosine-metabolizing enzyme. Importantly, hippocampal tissue resected from patients with medically intractable epilepsy demonstrated increased adenosine kinase. We therefore conclude that adenosine deficiency may be relevant to human epilepsy and that KD can reduce seizures by increasing A1R-mediated inhibition.
Figures


Comment in
-
Adenosine: front and center in linking nutrition and metabolism to neuronal activity.J Clin Invest. 2011 Jul;121(7):2548-50. doi: 10.1172/JCI58391. Epub 2011 Jun 23. J Clin Invest. 2011. PMID: 21701073 Free PMC article.
Similar articles
-
Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats.Neuropharmacology. 2015 Dec;99:500-9. doi: 10.1016/j.neuropharm.2015.08.007. Epub 2015 Aug 6. Neuropharmacology. 2015. PMID: 26256422 Free PMC article.
-
Ketogenic diet sensitizes glucose control of hippocampal excitability.J Lipid Res. 2014 Nov;55(11):2254-60. doi: 10.1194/jlr.M046755. Epub 2014 Aug 28. J Lipid Res. 2014. PMID: 25170119 Free PMC article.
-
Adenosine: front and center in linking nutrition and metabolism to neuronal activity.J Clin Invest. 2011 Jul;121(7):2548-50. doi: 10.1172/JCI58391. Epub 2011 Jun 23. J Clin Invest. 2011. PMID: 21701073 Free PMC article.
-
New insights into the mechanisms of the ketogenic diet.Curr Opin Neurol. 2017 Apr;30(2):187-192. doi: 10.1097/WCO.0000000000000432. Curr Opin Neurol. 2017. PMID: 28141738 Free PMC article. Review.
-
Metabolism and epilepsy: Ketogenic diets as a homeostatic link.Brain Res. 2019 Jan 15;1703:26-30. doi: 10.1016/j.brainres.2018.05.049. Epub 2018 Jun 6. Brain Res. 2019. PMID: 29883626 Free PMC article. Review.
Cited by
-
The hydroxycarboxylic acid receptor HCA2 is required for the protective effect of ketogenic diet in epilepsy.Pharmacol Res Perspect. 2024 Dec;12(6):e70026. doi: 10.1002/prp2.70026. Pharmacol Res Perspect. 2024. PMID: 39439218 Free PMC article.
-
Opiate-induced changes in brain adenosine levels and narcotic drug responses.Neuroscience. 2013 Jan 3;228:235-42. doi: 10.1016/j.neuroscience.2012.10.031. Epub 2012 Oct 22. Neuroscience. 2013. PMID: 23098802 Free PMC article.
-
Adenosine: a fundamental factor formed from Fatty feasts for fighting fits?Epilepsy Curr. 2012 Jan;12(1):19-21. doi: 10.5698/1535-7511-12.1.19. Epilepsy Curr. 2012. PMID: 22368522 Free PMC article. No abstract available.
-
The sound of noninvasive seizure control.Epilepsy Curr. 2011 Nov;11(6):196-7. doi: 10.5698/1535-7511-11.6.196. Epilepsy Curr. 2011. PMID: 22130328 Free PMC article. No abstract available.
-
Metabolic Aspects of Adenosine Functions in the Brain.Front Pharmacol. 2021 May 14;12:672182. doi: 10.3389/fphar.2021.672182. eCollection 2021. Front Pharmacol. 2021. PMID: 34054547 Free PMC article. Review.
References
-
- Dunwiddie TV, Worth T. Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J Pharmacol Exp Ther. 1982;220(1):70–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

