Poliovirus switches to an eIF2-independent mode of translation during infection
- PMID: 21697471
- PMCID: PMC3165854
- DOI: 10.1128/JVI.00792-11
Poliovirus switches to an eIF2-independent mode of translation during infection
Abstract
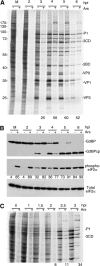
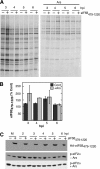
Inhibition of translation is an integral component of the innate antiviral response and is largely accomplished via interferon-activated phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α). To successfully infect a host, a virus must overcome this blockage by either controlling eIF2α phosphorylation or by utilizing a noncanonical mode of translation initiation. Here we show that enterovirus RNA is sensitive to translation inhibition resulting from eIF2α phosphorylation, but it becomes resistant as infection progresses. Further, we show that the cleavage of initiation factor eIF5B during enteroviral infection, along with the viral internal ribosome entry site, plays a role in mediating viral translation under conditions that are nonpermissive for host cell translation. Together, these results provide a mechanism by which enteroviruses evade the antiviral response and provide insight into a noncanonical mechanism of translation initiation.
Figures




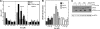
Similar articles
-
Eukaryotic initiation factor 5B: a new player for the anti-hepatitis C virus effect of ribavirin?Med Hypotheses. 2012 Oct;79(4):471-3. doi: 10.1016/j.mehy.2012.06.026. Epub 2012 Jul 21. Med Hypotheses. 2012. PMID: 22824093
-
Eukaryotic translation initiation machinery can operate in a bacterial-like mode without eIF2.Nat Struct Mol Biol. 2008 Aug;15(8):836-41. doi: 10.1038/nsmb.1445. Epub 2008 Jul 6. Nat Struct Mol Biol. 2008. PMID: 18604219
-
Cleavage of eukaryotic initiation factor eIF5B by enterovirus 3C proteases.Virology. 2008 Aug 15;378(1):118-22. doi: 10.1016/j.virol.2008.05.019. Epub 2008 Jun 24. Virology. 2008. PMID: 18572216 Free PMC article.
-
Hepatitis C Virus Translation Regulation.Int J Mol Sci. 2020 Mar 27;21(7):2328. doi: 10.3390/ijms21072328. Int J Mol Sci. 2020. PMID: 32230899 Free PMC article. Review.
-
Viral strategies to subvert the mammalian translation machinery.Prog Mol Biol Transl Sci. 2009;90:313-67. doi: 10.1016/S1877-1173(09)90009-6. Epub 2009 Oct 27. Prog Mol Biol Transl Sci. 2009. PMID: 20374746 Free PMC article. Review.
Cited by
-
Diversion of stress granules and P-bodies during viral infection.Virology. 2013 Feb 20;436(2):255-67. doi: 10.1016/j.virol.2012.11.017. Epub 2013 Jan 3. Virology. 2013. PMID: 23290869 Free PMC article. Review.
-
eIF5B drives integrated stress response-dependent translation of PD-L1 in lung cancer.Nat Cancer. 2020 May;1(5):533-545. doi: 10.1038/s43018-020-0056-0. Epub 2020 Apr 20. Nat Cancer. 2020. PMID: 32984844 Free PMC article.
-
Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1.mBio. 2015 Mar 17;6(2):e02486. doi: 10.1128/mBio.02486-14. mBio. 2015. PMID: 25784705 Free PMC article.
-
Animal virus schemes for translation dominance.Curr Opin Virol. 2011 Nov;1(5):363-72. doi: 10.1016/j.coviro.2011.10.009. Curr Opin Virol. 2011. PMID: 22319551 Free PMC article. Review.
-
Puumala and Andes Orthohantaviruses Cause Transient Protein Kinase R-Dependent Formation of Stress Granules.J Virol. 2020 Jan 17;94(3):e01168-19. doi: 10.1128/JVI.01168-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31723021 Free PMC article.
References
-
- Anderson P., Kedersha N. 2009. Stress granules. Curr. Biol. 19:R397–R398 - PubMed
-
- Atlas R., Behar L., Elliott E., Ginzburg I. 2004. The insulin-like growth factor mRNA binding-protein IMP-1 and the Ras-regulatory protein G3BP associate with tau mRNA and HuD protein in differentiated P19 neuronal cells. J. Neurochem. 89:613–626 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

