Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA
- PMID: 21690403
- PMCID: PMC3131353
- DOI: 10.1073/pnas.1101939108
Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA
Abstract
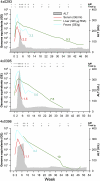
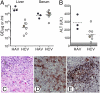
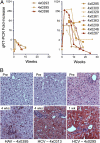
Hepatitis A virus (HAV) is an hepatotropic human picornavirus that is associated only with acute infection. Its pathogenesis is not well understood because there are few studies in animal models using modern methodologies. We characterized HAV infections in three chimpanzees, quantifying viral RNA by quantitative RT-PCR and examining critical aspects of the innate immune response including intrahepatic IFN-stimulated gene expression. We compared these infection profiles with similar studies of chimpanzees infected with hepatitis C virus (HCV), an hepatotropic flavivirus that frequently causes persistent infection. Surprisingly, HAV-infected animals exhibited very limited induction of type I IFN-stimulated genes in the liver compared with chimpanzees with acute resolving HCV infection, despite similar levels of viremia and 100-fold greater quantities of viral RNA in the liver. Minimal IFN-stimulated gene 15 and IFIT1 responses peaked 1-2 wk after HAV challenge and then subsided despite continuing high hepatic viral RNA. An acute inflammatory response at 3-4 wk correlated with the appearance of virus-specific antibodies and apoptosis and proliferation of hepatocytes. Despite this, HAV RNA persisted in the liver for months, remaining present long after clearance from serum and feces and revealing dramatic differences in the kinetics of clearance in the three compartments. Viral RNA was detected in the liver for significantly longer (35 to >48 wk) than HCV RNA in animals with acute resolving HCV infection (10-20 wk). Collectively, these findings indicate that HAV is far stealthier than HCV early in the course of acute resolving infection. HAV infections represent a distinctly different paradigm in virus-host interactions within the liver.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Hepatitis A and hepatitis C viruses: divergent infection outcomes marked by similarities in induction and evasion of interferon responses.Semin Liver Dis. 2010 Nov;30(4):319-32. doi: 10.1055/s-0030-1267534. Epub 2010 Oct 19. Semin Liver Dis. 2010. PMID: 20960373 Review.
-
In vivo study of the HC-TN strain of hepatitis C virus recovered from a patient with fulminant hepatitis: RNA transcripts of a molecular clone (pHC-TN) are infectious in chimpanzees but not in Huh7.5 cells.J Virol. 2007 Jul;81(13):7208-19. doi: 10.1128/JVI.01774-06. Epub 2007 Apr 4. J Virol. 2007. PMID: 17409145 Free PMC article.
-
MAVS-dependent host species range and pathogenicity of human hepatitis A virus.Science. 2016 Sep 30;353(6307):1541-1545. doi: 10.1126/science.aaf8325. Epub 2016 Sep 15. Science. 2016. PMID: 27633528 Free PMC article.
-
Macrophage Depletion Reactivates Fecal Virus Shedding following Resolution of Acute Hepatitis A in Ifnar1-/- Mice.J Virol. 2022 Dec 14;96(23):e0149622. doi: 10.1128/jvi.01496-22. Epub 2022 Nov 10. J Virol. 2022. PMID: 36354341 Free PMC article.
-
[Hepatitis A virus infection. A review].Praxis (Bern 1994). 2003 Oct 1;92(40):1659-73. doi: 10.1024/0369-8394.92.40.1659. Praxis (Bern 1994). 2003. PMID: 14579471 Review. German.
Cited by
-
A single mutation in the glycophorin A binding site of hepatitis A virus enhances virus clearance from the blood and results in a lower fitness variant.J Virol. 2012 Aug;86(15):7887-95. doi: 10.1128/JVI.00707-12. Epub 2012 May 16. J Virol. 2012. PMID: 22593170 Free PMC article.
-
Novel perspectives for hepatitis A virus therapy revealed by comparative analysis of hepatitis C virus and hepatitis A virus RNA replication.Hepatology. 2015 Aug;62(2):397-408. doi: 10.1002/hep.27847. Epub 2015 May 20. Hepatology. 2015. PMID: 25866017 Free PMC article.
-
The infectivity and pathogenicity of hepatitis A virus live-attenuated vaccine strain H2 in type I interferon receptor-deficient mice.Virol Sin. 2022 Oct;37(5):740-745. doi: 10.1016/j.virs.2022.07.009. Epub 2022 Jul 19. Virol Sin. 2022. PMID: 35863604 Free PMC article.
-
Adaptive Immune Responses in Hepatitis A Virus and Hepatitis E Virus Infections.Cold Spring Harb Perspect Med. 2019 Sep 3;9(9):a033472. doi: 10.1101/cshperspect.a033472. Cold Spring Harb Perspect Med. 2019. PMID: 29844218 Free PMC article. Review.
-
Hepatitis A Virus and Hepatitis E Virus: Emerging and Re-Emerging Enterically Transmitted Hepatitis Viruses.Cold Spring Harb Perspect Med. 2019 Jun 3;9(6):a031823. doi: 10.1101/cshperspect.a031823. Cold Spring Harb Perspect Med. 2019. PMID: 29735577 Free PMC article. Review.
References
-
- Feng Z, Lemon SM. Pathogenesis of hepatitis A virus infection. In: Domingo E, Ehrenfeld E, Roos R, editors. The Picornaviruses: Molecular Biology, Evolution and Pathogenesis. Washington, DC: American Society for Microbiology Press; 2010. pp. 383–396.
-
- Gust ID, Feinstone SM. Hepatitis A. Prog Liver Dis. 1990;9:371–378. - PubMed
-
- Wong DC, Purcell RH, Rosen L. Prevalence of antibody to hepatitis A and hepatitis B viruses in selected populations of the South Pacific. Am J Epidemiol. 1979;110:227–236. - PubMed
-
- Skinhoj P, Mikkelsen F, Hollinger FB. Hepatitis A in Greenland: Importance of specific antibody testing in epidemiologic surveillance. Am J Epidemiol. 1977;105:140–147. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 OD011095/OD/NIH HHS/United States
- C06 RR12087/RR/NCRR NIH HHS/United States
- C06 RR016228/RR/NCRR NIH HHS/United States
- AI028433/AI/NIAID NIH HHS/United States
- R37 AI028433/AI/NIAID NIH HHS/United States
- U19 AI48231/AI/NIAID NIH HHS/United States
- U19 AI040035/AI/NIAID NIH HHS/United States
- R01 RR006555/RR/NCRR NIH HHS/United States
- U19 AI40035/AI/NIAID NIH HHS/United States
- R21 AI081058/AI/NIAID NIH HHS/United States
- C06 RR012087/RR/NCRR NIH HHS/United States
- R37 AI047367/AI/NIAID NIH HHS/United States
- RR16228/RR/NCRR NIH HHS/United States
- P51 RR013986/RR/NCRR NIH HHS/United States
- RR006555/RR/NCRR NIH HHS/United States
- R01 AI028433/AI/NIAID NIH HHS/United States
- P51 RR13986/RR/NCRR NIH HHS/United States
- U19 AI048231/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

