Rab11-FIP2 influences multiple components of the endosomal system in polarized MDCK cells
- PMID: 21686255
- PMCID: PMC3116584
- DOI: 10.4161/cl.1.2.15289
Rab11-FIP2 influences multiple components of the endosomal system in polarized MDCK cells
Abstract
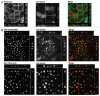
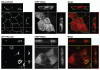
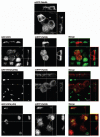
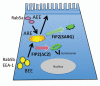
The Rab11 Family Interacting Proteins (Rab11-FIPs) are hypothesized to regulate sequential steps in the apical recycling and transcytotic pathways of polarized epithelial cells. Previous studies have suggested that Rab11-FIP proteins assemble into multi-protein complexes regulating plasma membrane recycling. Rab11-FIP2 interacts with both myosin Vb and Rab11. Recent investigations have noted that that Rab11-FIP2 mutants [Rab11-FIP2(129-512), also designated Rab11-FIP2(ΔC2) and Rab11-FIP2(S229A, R413G), also designated Rab11-FIP2(SARG)], are potent inhibitors of transcytosis in polarized MDCK cells. Interestingly, Rab11-FIP2(ΔC2), but not Rab11-FIP2(SARG), also altered the morphology of the EEA-1 positive early endosomal compartment. These findings suggested that Rab11-FIP2 mutants could differentiate different points along the recycling pathway. We therefore sought to investigate whether Rab11-FIP2 is a general regulator of the early endosomal system. Both Rab11-FIP2 mutants altered the localization and co-localized with dynein heavy chain. In contrast, both clathrin heavy chain and AP-1 accumulated with membranes containing Rab11-FIP2(SARG), but not with Rab11-FIP2(ΔC2). Expression of Rab11-FIP2(ΔC2), but not Rab11-FIP2(SARG), caused clustering of early endosomal markers Rab5b, Epsin 4 and IQGAP1, around a collapsed Rab11-FIP2 containing membranous cisternum. Interestingly, neither Rab11-FIP2 mutant had any effect on the distribution of Rab5a, a classical early endosome marker. The results support the view that Rab11-FIP2 may influence microtubule-dependent centripetal movement of subsets of early endosomes as well as processing through the common and recycling endosomal systems.
Figures





Similar articles
-
Rab11-FIP2 regulates differentiable steps in transcytosis.Am J Physiol Cell Physiol. 2007 Sep;293(3):C1059-72. doi: 10.1152/ajpcell.00078.2007. Epub 2007 Jul 11. Am J Physiol Cell Physiol. 2007. PMID: 17626244
-
Rab11-FIP2 functions in transferrin recycling and associates with endosomal membranes via its COOH-terminal domain.J Biol Chem. 2002 Jul 26;277(30):27193-9. doi: 10.1074/jbc.M200757200. Epub 2002 May 6. J Biol Chem. 2002. PMID: 11994279
-
Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling.J Biol Chem. 2002 Dec 27;277(52):50415-21. doi: 10.1074/jbc.M209270200. Epub 2002 Oct 21. J Biol Chem. 2002. PMID: 12393859
-
The dynamic Rab11-FIPs.Biochem Soc Trans. 2009 Oct;37(Pt 5):1032-6. doi: 10.1042/BST0371032. Biochem Soc Trans. 2009. PMID: 19754446 Review.
-
Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity.Histol Histopathol. 2009 Sep;24(9):1171-80. doi: 10.14670/HH-24.1171. Histol Histopathol. 2009. PMID: 19609864 Free PMC article. Review.
Cited by
-
Distinct patterns of phosphatidylserine localization within the Rab11a-containing recycling system.Cell Logist. 2014 Apr 3;4:e28680. doi: 10.4161/cl.28680. eCollection 2014. Cell Logist. 2014. PMID: 25210648 Free PMC article.
-
Rab11-family interacting proteins define spatially and temporally distinct regions within the dynamic Rab11a-dependent recycling system.Mol Biol Cell. 2013 Mar;24(5):643-58. doi: 10.1091/mbc.E12-09-0659. Epub 2013 Jan 2. Mol Biol Cell. 2013. PMID: 23283983 Free PMC article.
-
Sorting it out: regulation of exosome loading.Semin Cancer Biol. 2014 Oct;28:3-13. doi: 10.1016/j.semcancer.2014.04.009. Epub 2014 Apr 23. Semin Cancer Biol. 2014. PMID: 24769058 Free PMC article. Review.
-
Rab11-FIP1 phosphorylation by MARK2 regulates polarity in MDCK cells.Cell Logist. 2017 Jan 9;7(1):e1271498. doi: 10.1080/21592799.2016.1271498. eCollection 2017. Cell Logist. 2017. PMID: 28396819 Free PMC article.
-
Coordinated regulation of caveolin-1 and Rab11a in apical recycling compartments of polarized epithelial cells.Exp Cell Res. 2012 Jan 15;318(2):103-13. doi: 10.1016/j.yexcr.2011.10.010. Epub 2011 Oct 20. Exp Cell Res. 2012. PMID: 22036648 Free PMC article.
References
-
- Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol Gastrointest Liver Physiol. 1996;270:515–525. - PubMed
-
- Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized madin-darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. - PubMed
-
- Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, et al. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–39075. - PubMed
-
- Wallace DM, Lindsay AJ, Hendrick AG, McCaffrey MW. The novel Rab11-FIP/Rip/RCP family of proteins displays extensive homo- and hetero-interacting abilities. Biochem Biophys Res Commun. 2002;292:909–915. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
