Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm
- PMID: 21683865
- PMCID: PMC3148026
- DOI: 10.1016/j.humpath.2011.03.003
Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm
Abstract
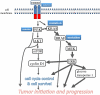
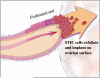
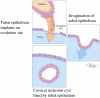
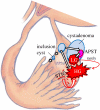
Recent morphologic, immunohistochemical, and molecular genetic studies have led to the development of a new paradigm for the pathogenesis and origin of epithelial ovarian cancer based on a dualistic model of carcinogenesis that divides epithelial ovarian cancer into 2 broad categories designated types I and II. Type I tumors comprise low-grade serous, low-grade endometrioid, clear cell and mucinous carcinomas, and Brenner tumors. They are generally indolent, present in stage I (tumor confined to the ovary), and are characterized by specific mutations, including KRAS, BRAF, ERBB2, CTNNB1, PTEN, PIK3CA, ARID1A, and PPP2R1A, which target specific cell signaling pathways. Type I tumors rarely harbor TP53 mutations and are relatively stable genetically. Type II tumors comprise high-grade serous, high-grade endometrioid, malignant mixed mesodermal tumors (carcinosarcomas), and undifferentiated carcinomas. They are aggressive, present in advanced stage, and have a very high frequency of TP53 mutations but rarely harbor the mutations detected in type I tumors. In addition, type II tumors have molecular alterations that perturb expression of BRCA either by mutation of the gene or by promoter methylation. A hallmark of these tumors is that they are genetically highly unstable. Recent studies strongly suggest that fallopian tube epithelium (benign or malignant) that implants on the ovary is the source of low-grade and high-grade serous carcinoma rather than the ovarian surface epithelium as previously believed. Similarly, it is widely accepted that endometriosis is the precursor of endometrioid and clear cell carcinomas and, as endometriosis, is thought to develop from retrograde menstruation; these tumors can also be regarded as involving the ovary secondarily. The origin of mucinous and transitional cell (Brenner) tumors is still not well established, although recent data suggest a possible origin from transitional epithelial nests located in paraovarian locations at the tuboperitoneal junction. Thus, it now appears that type I and type II ovarian tumors develop independently along different molecular pathways and that both types develop outside the ovary and involve it secondarily. If this concept is confirmed, it leads to the conclusion that the only true primary ovarian neoplasms are gonadal stromal and germ cell tumors analogous to testicular tumors. This new paradigm of ovarian carcinogenesis has important clinical implications. By shifting the early events of ovarian carcinogenesis to the fallopian tube and endometrium instead of the ovary, prevention approaches, for example, salpingectomy with ovarian conservation, may play an important role in reducing the burden of ovarian cancer while preserving hormonal function and fertility.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory.Am J Surg Pathol. 2010 Mar;34(3):433-43. doi: 10.1097/PAS.0b013e3181cf3d79. Am J Surg Pathol. 2010. PMID: 20154587 Free PMC article.
-
Origin and molecular pathogenesis of ovarian high-grade serous carcinoma.Ann Oncol. 2013 Dec;24 Suppl 10:x16-21. doi: 10.1093/annonc/mdt463. Ann Oncol. 2013. PMID: 24265397 Review.
-
Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications.Int J Gynecol Pathol. 2008 Apr;27(2):151-60. doi: 10.1097/PGP.0b013e318161e4f5. Int J Gynecol Pathol. 2008. PMID: 18317228 Free PMC article. Review.
-
Precursors and pathogenesis of ovarian carcinoma.Pathology. 2013 Apr;45(3):229-42. doi: 10.1097/PAT.0b013e32835f2264. Pathology. 2013. PMID: 23478230 Review.
-
Morphologic, Immunophenotypic, and Molecular Features of Epithelial Ovarian Cancer.Oncology (Williston Park). 2016 Feb;30(2):166-76. Oncology (Williston Park). 2016. PMID: 26892153 Review.
Cited by
-
Preventing ovarian cancer by salpingectomy.Curr Oncol. 2013 Jun;20(3):139-42. doi: 10.3747/co.20.1613. Curr Oncol. 2013. PMID: 23737679 Free PMC article. No abstract available.
-
Reproductive characteristics in relation to ovarian cancer risk by histologic pathways.Hum Reprod. 2013 May;28(5):1406-17. doi: 10.1093/humrep/des466. Epub 2013 Jan 12. Hum Reprod. 2013. PMID: 23315066 Free PMC article.
-
Immunobiology of human mucin 1 in a preclinical ovarian tumor model.Oncogene. 2013 Aug 8;32(32):3664-75. doi: 10.1038/onc.2012.397. Epub 2012 Sep 10. Oncogene. 2013. PMID: 22964632 Free PMC article.
-
Cytokeratin and protein expression patterns in squamous cell carcinoma of the oral cavity provide evidence for two distinct pathogenetic pathways.Oncol Lett. 2016 Jul;12(1):107-113. doi: 10.3892/ol.2016.4588. Epub 2016 May 16. Oncol Lett. 2016. PMID: 27347109 Free PMC article.
-
The PI3K/Akt/mTOR pathway in ovarian cancer: therapeutic opportunities and challenges.Chin J Cancer. 2015 Jan;34(1):4-16. doi: 10.5732/cjc.014.10289. Chin J Cancer. 2015. PMID: 25556614 Free PMC article. Review.
References
-
- Kuhn TS. The Structure of Scientific Revolutions. 3rd ed. University of Chicago Press; 1996. enlarged.
-
- Geist SH. Ovarian Tumors. Paul B. Hoeber, Inc; New York: 1942.
-
- Frank RT. Gynecological and Obstetrical Pathology. D Appleton and Co; New York: 1931.
-
- Gemma B. Atlas of Ovarian Tumors. Grune & Stratton; New York: 1943.
-
- Hertig AT, Gore H. Atlas of Tumor Pathology, Section IX-Fascicle 3,Tumors of the Female Sex Organs Part 3 Tumors of the Ovary and Fallopian Tube. Armed Forces Institute of Pathology; Washington DC: 1961.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

