Monocyte and macrophage dynamics during atherogenesis
- PMID: 21677293
- PMCID: PMC3133596
- DOI: 10.1161/ATVBAHA.110.221127
Monocyte and macrophage dynamics during atherogenesis
Abstract
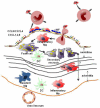
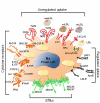
Vascular inflammation is associated with and in large part driven by changes in the leukocyte compartment of the vessel wall. Here, we focus on monocyte influx during atherosclerosis, the most common form of vascular inflammation. Although the arterial wall contains a large number of resident macrophages and some resident dendritic cells, atherosclerosis drives a rapid influx of inflammatory monocytes (Ly-6C(+) in mice) and other monocytes (Ly-6C(-) in mice, also known as patrolling monocytes). Once in the vessel wall, Ly-6C(+) monocytes differentiate to a phenotype consistent with inflammatory macrophages and inflammatory dendritic cells. The phenotype of these cells is modulated by lipid uptake, Toll-like receptor ligands, hematopoietic growth factors, cytokines, and chemokines. In addition to newly recruited macrophages, it is likely that resident macrophages also change their phenotype. Monocyte-derived inflammatory macrophages have a short half-life. After undergoing apoptosis, they may be taken up by surrounding macrophages or, if the phagocytic capacity is overwhelmed, can undergo secondary necrosis, a key event in forming the necrotic core of atherosclerotic lesions. In this review, we discuss these and other processes associated with monocytic cell dynamics in the vascular wall and their role in the initiation and progression of atherosclerosis.
Figures
Similar articles
-
The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis.Immunobiology. 2013 Nov;218(11):1376-84. doi: 10.1016/j.imbio.2013.06.005. Epub 2013 Jun 19. Immunobiology. 2013. PMID: 23886694
-
Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation.Thromb Haemost. 2011 Nov;106(5):763-71. doi: 10.1160/TH11-05-0320. Epub 2011 Sep 22. Thromb Haemost. 2011. PMID: 21947328 Review.
-
Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice.Circulation. 2009 Nov 10;120(19):1893-902. doi: 10.1161/CIRCULATIONAHA.109.866889. Epub 2009 Oct 26. Circulation. 2009. PMID: 19858416 Free PMC article.
-
Monocyte fate in atherosclerosis.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):272-9. doi: 10.1161/ATVBAHA.114.303565. Epub 2014 Dec 23. Arterioscler Thromb Vasc Biol. 2015. PMID: 25538208 Review.
-
IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis.J Immunol. 2014 Nov 1;193(9):4344-55. doi: 10.4049/jimmunol.1400181. Epub 2014 Sep 26. J Immunol. 2014. PMID: 25261478 Free PMC article.
Cited by
-
Transcriptome-Wide Analysis Reveals Modulation of Human Macrophage Inflammatory Phenotype Through Alternative Splicing.Arterioscler Thromb Vasc Biol. 2016 Jul;36(7):1434-47. doi: 10.1161/ATVBAHA.116.307573. Epub 2016 May 26. Arterioscler Thromb Vasc Biol. 2016. PMID: 27230130 Free PMC article.
-
Local and systemic factors associated with quantitative stiffness of carotid plaque.Acta Neurochir (Wien). 2024 Jan 30;166(1):54. doi: 10.1007/s00701-024-05952-z. Acta Neurochir (Wien). 2024. PMID: 38289409
-
NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis.Circ Res. 2012 Feb 3;110(3):416-27. doi: 10.1161/CIRCRESAHA.111.253377. Epub 2011 Dec 22. Circ Res. 2012. PMID: 22194622 Free PMC article.
-
P2X4 deficiency reduces atherosclerosis and plaque inflammation in mice.Sci Rep. 2022 Feb 18;12(1):2801. doi: 10.1038/s41598-022-06706-6. Sci Rep. 2022. PMID: 35181718 Free PMC article.
-
Rapamycin and FTY720 Alleviate Atherosclerosis by Cross Talk of Macrophage Polarization and Autophagy.Biomed Res Int. 2018 Dec 6;2018:1010248. doi: 10.1155/2018/1010248. eCollection 2018. Biomed Res Int. 2018. PMID: 30627532 Free PMC article.
References
-
- Cybulsky MI, Won D, Haidari M. Leukocyte recruitment to atherosclerotic lesions. Can J Cardiol. 2004;20(Suppl B):24B–8B. - PubMed
-
- Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin Immunopathol. 2009;31:35–47. - PubMed
-
- Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

