Methionine sulfoxide reductase A is a stereospecific methionine oxidase
- PMID: 21670260
- PMCID: PMC3127874
- DOI: 10.1073/pnas.1101275108
Methionine sulfoxide reductase A is a stereospecific methionine oxidase
Abstract
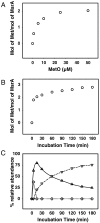
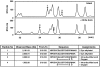
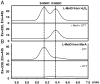
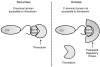
Methionine sulfoxide reductase A (MsrA) catalyzes the reduction of methionine sulfoxide to methionine and is specific for the S epimer of methionine sulfoxide. The enzyme participates in defense against oxidative stresses by reducing methionine sulfoxide residues in proteins back to methionine. Because oxidation of methionine residues is reversible, this covalent modification could also function as a mechanism for cellular regulation, provided there exists a stereospecific methionine oxidase. We show that MsrA itself is a stereospecific methionine oxidase, producing S-methionine sulfoxide as its product. MsrA catalyzes its own autooxidation as well as oxidation of free methionine and methionine residues in peptides and proteins. When functioning as a reductase, MsrA fully reverses the oxidations which it catalyzes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Stereospecific oxidation of calmodulin by methionine sulfoxide reductase A.Free Radic Biol Med. 2013 Aug;61:257-64. doi: 10.1016/j.freeradbiomed.2013.04.004. Epub 2013 Apr 11. Free Radic Biol Med. 2013. PMID: 23583331 Free PMC article.
-
Methionine Sulfoxide Reductase A (MsrA) and Its Function in Ubiquitin-Like Protein Modification in Archaea.mBio. 2017 Sep 5;8(5):e01169-17. doi: 10.1128/mBio.01169-17. mBio. 2017. PMID: 28874471 Free PMC article.
-
Drosophila methionine sulfoxide reductase A (MSRA) lacks methionine oxidase activity.Free Radic Biol Med. 2019 Feb 1;131:154-161. doi: 10.1016/j.freeradbiomed.2018.12.001. Epub 2018 Dec 4. Free Radic Biol Med. 2019. PMID: 30529269 Free PMC article.
-
The discovery of methionine sulfoxide reductase enzymes: An historical account and future perspectives.Biofactors. 2015 May 6;41(3):135-52. doi: 10.1002/biof.1214. Epub 2015 May 12. Biofactors. 2015. PMID: 25963551 Review.
-
Recharging oxidative protein repair: catalysis by methionine sulfoxide reductases towards their amino acid, protein, and model substrates.Biochemistry (Mosc). 2012 Oct;77(10):1097-107. doi: 10.1134/S0006297912100021. Biochemistry (Mosc). 2012. PMID: 23157290 Review.
Cited by
-
A low pKa cysteine at the active site of mouse methionine sulfoxide reductase A.J Biol Chem. 2012 Jul 20;287(30):25596-601. doi: 10.1074/jbc.M112.369116. Epub 2012 Jun 1. J Biol Chem. 2012. PMID: 22661719 Free PMC article.
-
Methionine sulfoxide reductase B3 requires resolving cysteine residues for full activity and can act as a stereospecific methionine oxidase.Biochem J. 2018 Feb 28;475(4):827-838. doi: 10.1042/BCJ20170929. Biochem J. 2018. PMID: 29420254 Free PMC article.
-
Methionine biosynthetic genes and methionine sulfoxide reductase A are required for Rhizoctonia solani AG1-IA to cause sheath blight disease in rice.Microb Biotechnol. 2024 Apr;17(4):e14441. doi: 10.1111/1751-7915.14441. Microb Biotechnol. 2024. PMID: 38568774 Free PMC article.
-
Multi-scale computational enzymology: enhancing our understanding of enzymatic catalysis.Int J Mol Sci. 2013 Dec 31;15(1):401-22. doi: 10.3390/ijms15010401. Int J Mol Sci. 2013. PMID: 24384841 Free PMC article. Review.
-
A Methionine Residue Promotes Hyperoxidation of the Catalytic Cysteine of Mouse Methionine Sulfoxide Reductase A.Biochemistry. 2016 Jun 28;55(25):3586-93. doi: 10.1021/acs.biochem.6b00180. Epub 2016 Jun 14. Biochemistry. 2016. PMID: 27259041 Free PMC article.
References
-
- Toennies G, Kolb JJ. Methionine studies. J Biol Chem. 1939;128:399–405.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

