Ubiquitin-independent proteasomal degradation during oncogenic viral infections
- PMID: 21664948
- PMCID: PMC3193896
- DOI: 10.1016/j.bbcan.2011.05.005
Ubiquitin-independent proteasomal degradation during oncogenic viral infections
Abstract
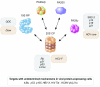
Most eukaryotic proteins destined for imminent destruction are first tagged with a chain of ubiquitin molecules and are subsequently dismantled by the proteasome. Ubiquitin-independent degradation of substrates by the proteasome, however, also occurs. The number of documented proteasome-dependent, ubiquitin-independent degradation events remains relatively small but continues to grow. Proteins involved in oncogenesis and tumor suppression make up the majority of the known cases for this type of protein destruction. Provocatively, viruses with confirmed or suspected oncogenic properties are also prominent participants in the pantheon of ubiquitin-independent proteasomal degradation events. In this review, we identify and describe examples of proteasome-dependent, ubiquitin-independent protein degradation that occur during tumor virus infections, speculate why this type of protein destruction may be preferred during oncogenesis, and argue that this uncommon type of protein turnover represents a prime target for antiviral and anticancer therapeutics.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Ubiquitin-independent degradation: lessons from the p53 model.Isr Med Assoc J. 2006 Apr;8(4):229-32. Isr Med Assoc J. 2006. PMID: 16671355 Review.
-
Ubiquitin-independent proteasomal degradation.Biochim Biophys Acta. 2014 Jan;1843(1):216-21. doi: 10.1016/j.bbamcr.2013.05.008. Epub 2013 May 14. Biochim Biophys Acta. 2014. PMID: 23684952 Free PMC article. Review.
-
[Proteasomal degradation: from addressing of substrates to therapeutical perspectives].Med Sci (Paris). 2005 Feb;21(2):141-9. doi: 10.1051/medsci/2005212141. Med Sci (Paris). 2005. PMID: 15691484 Review. French.
-
[Degradation of proteins by ubiquitin proteasome pathway].Klin Onkol. 2013;26(4):251-6. doi: 10.14735/amko2013251. Klin Onkol. 2013. PMID: 23961855 Review. Czech.
-
Ubiquitin-independent degradation and its implication in cancer.Future Oncol. 2005 Oct;1(5):567-70. doi: 10.2217/14796694.1.5.567. Future Oncol. 2005. PMID: 16556032 Review. No abstract available.
Cited by
-
Ubiquitin-independent proteasomal degradation of tumor suppressors by human cytomegalovirus pp71 requires the 19S regulatory particle.J Virol. 2013 Apr;87(8):4665-71. doi: 10.1128/JVI.03301-12. Epub 2013 Feb 13. J Virol. 2013. PMID: 23408605 Free PMC article.
-
Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples.J Virol. 2012 Jan;86(2):854-64. doi: 10.1128/JVI.06097-11. Epub 2011 Nov 16. J Virol. 2012. PMID: 22090104 Free PMC article.
-
The Proteasome Subunit Rpn8 Interacts with the Small Nucleolar RNA Protein (snoRNP) Assembly Protein Pih1 and Mediates Its Ubiquitin-independent Degradation in Saccharomyces cerevisiae.J Biol Chem. 2016 May 27;291(22):11761-75. doi: 10.1074/jbc.M115.702043. Epub 2016 Apr 6. J Biol Chem. 2016. PMID: 27053109 Free PMC article.
-
Regulation of cell surface protease receptor S100A10 by retinoic acid therapy in acute promyelocytic leukemia (APL)☆.Cell Death Dis. 2018 Sep 11;9(9):920. doi: 10.1038/s41419-018-0954-6. Cell Death Dis. 2018. PMID: 30206209 Free PMC article.
-
Evaluation of the NRF1-proteasome axis as a therapeutic target in breast cancer.Sci Rep. 2023 Sep 22;13(1):15843. doi: 10.1038/s41598-023-43121-x. Sci Rep. 2023. PMID: 37739987 Free PMC article.
References
-
- Chondrogianni N, Gonos ES. Proteasome function determines cellular homeostasis and the rate of aging. Adv Exp Med Biol. 2010;694:38–46. - PubMed
-
- Lecker SH, Goldberg AL, Mitch WE. Protein Degradation by the Ubiquitin–Proteasome Pathway in Normal and Disease States. Journal of the American Society of Nephrology. 2006;17:1807–1819. - PubMed
-
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

