The human commensal Bacteroides fragilis binds intestinal mucin
- PMID: 21664470
- PMCID: PMC3163789
- DOI: 10.1016/j.anaerobe.2011.05.017
The human commensal Bacteroides fragilis binds intestinal mucin
Abstract
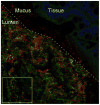
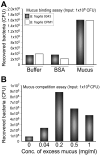
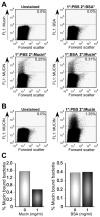
The mammalian gastrointestinal tract harbors a vast microbial ecosystem, known as the microbiota, which benefits host biology. Bacteroides fragilis is an important anaerobic gut commensal of humans that prevents and cures intestinal inflammation. We wished to elucidate aspects of gut colonization employed by B. fragilis. Fluorescence in situ hybridization was performed on colonic tissue sections from B. fragilis and Escherichia coli dual-colonized gnotobiotic mice. Epifluorescence imaging reveals that both E. coli and B. fragilis are found in the lumen of the colon, but only B. fragilis is found in the mucosal layer. This observation suggests that physical association with intestinal mucus could be a possible mechanism of gut colonization by B. fragilis. We investigated this potential interaction using an in vitro mucus binding assay and show here that B. fragilis binds to murine colonic mucus. We further demonstrate that B. fragilis specifically and quantitatively binds to highly purified mucins (the major constituent in intestinal mucus) using flow cytometry analysis of fluorescently labeled purified murine and porcine mucins. These results suggest that interactions between B. fragilis and intestinal mucin may play a critical role during host-bacterial symbiosis.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Bacterial colonization factors control specificity and stability of the gut microbiota.Nature. 2013 Sep 19;501(7467):426-9. doi: 10.1038/nature12447. Epub 2013 Aug 18. Nature. 2013. PMID: 23955152 Free PMC article.
-
Spatially distinct physiology of Bacteroides fragilis within the proximal colon of gnotobiotic mice.Nat Microbiol. 2020 May;5(5):746-756. doi: 10.1038/s41564-020-0683-3. Epub 2020 Mar 9. Nat Microbiol. 2020. PMID: 32152589 Free PMC article.
-
The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota.Science. 2011 May 20;332(6032):974-7. doi: 10.1126/science.1206095. Epub 2011 Apr 21. Science. 2011. PMID: 21512004 Free PMC article.
-
Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis.J Clin Invest. 2014 Oct;124(10):4166-72. doi: 10.1172/JCI72334. Epub 2014 Aug 8. J Clin Invest. 2014. PMID: 25105360 Free PMC article. Review.
-
Intestinal mucus and their glycans: A habitat for thriving microbiota.Cell Host Microbe. 2023 Jul 12;31(7):1087-1100. doi: 10.1016/j.chom.2023.05.026. Cell Host Microbe. 2023. PMID: 37442097 Free PMC article. Review.
Cited by
-
Host/microbiota interactions in health and diseases-Time for mucosal microbiology!Mucosal Immunol. 2021 Sep;14(5):1006-1016. doi: 10.1038/s41385-021-00383-w. Epub 2021 Mar 26. Mucosal Immunol. 2021. PMID: 33772148 Free PMC article. Review.
-
Home, sweet home: how mucus accommodates our microbiota.FEBS J. 2021 Mar;288(6):1789-1799. doi: 10.1111/febs.15504. Epub 2020 Aug 14. FEBS J. 2021. PMID: 32755014 Free PMC article. Review.
-
The gut microbiome and colorectal cancer: a review of bacterial pathogenesis.J Gastrointest Oncol. 2018 Aug;9(4):769-777. doi: 10.21037/jgo.2018.04.07. J Gastrointest Oncol. 2018. PMID: 30151274 Free PMC article. Review.
-
Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management.Front Immunol. 2021 Dec 21;12:800879. doi: 10.3389/fimmu.2021.800879. eCollection 2021. Front Immunol. 2021. PMID: 34992611 Free PMC article. Review.
-
Microbiota-mediated colonization resistance against intestinal pathogens.Nat Rev Immunol. 2013 Nov;13(11):790-801. doi: 10.1038/nri3535. Epub 2013 Oct 7. Nat Rev Immunol. 2013. PMID: 24096337 Free PMC article. Review.
References
-
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. - PubMed
-
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. - PubMed
-
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

