Sonic hedgehog-induced functional recovery after myocardial infarction is enhanced by AMD3100-mediated progenitor-cell mobilization
- PMID: 21658566
- PMCID: PMC3117426
- DOI: 10.1016/j.jacc.2010.11.069
Sonic hedgehog-induced functional recovery after myocardial infarction is enhanced by AMD3100-mediated progenitor-cell mobilization
Abstract
Objectives: This study was designed to compare the effectiveness of Sonic hedgehog (Shh) gene transfer, AMD3100-induced progenitor-cell mobilization, and Shh-AMD3100 combination therapy for treatment of surgically induced myocardial infarction (MI) in mice.
Background: Shh gene transfer improves myocardial recovery by up-regulating angiogenic genes and enhancing the incorporation of bone marrow-derived progenitor cells (BMPCs) in infarcted myocardium. Here, we investigated whether the effectiveness of Shh gene therapy could be improved with AMD3100-induced progenitor-cell mobilization.
Methods: Gene expression and cell function were evaluated in cells cultured with medium collected from fibroblasts transfected with plasmids encoding human Shh (phShh). MI was induced in wild-type mice, in matrix metalloproteinase (MMP)-9 knockout mice, and in mice transplanted with bone marrow that expressed green-fluorescent protein. Mice were treated with 100 μg of phShh (administered intramyocardially), 5 mg/kg of AMD3100 (administered subcutaneously), or both; cardiac function was evaluated echocardiographically, and fibrosis, capillary density, and BMPC incorporation were evaluated immunohistochemically.
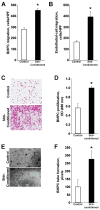
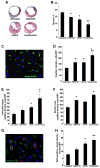
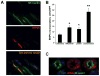
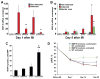
Results: phShh increased vascular endothelial growth factor and stromal cell-derived factor 1 expression in fibroblasts; the medium from phShh-transfected fibroblasts increased endothelial-cell migration and the migration, proliferation, and tube formation of BMPCs. Combination therapy enhanced cardiac functional recovery (i.e., left ventricular ejection fraction) in wild-type mice, but not in MMP-9 knockout mice, and was associated with less fibrosis, greater capillary density and smooth muscle-containing vessel density, and enhanced BMPC incorporation.
Conclusions: Combination therapy consisting of intramyocardial Shh gene transfer and AMD3100-induced progenitor-cell mobilization improves cardiac functional recovery after MI and is superior to either individual treatment for promoting therapeutic neovascularization.
Copyright © 2011 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice.JACC Cardiovasc Imaging. 2009 Jul;2(7):869-79. doi: 10.1016/j.jcmg.2009.04.008. JACC Cardiovasc Imaging. 2009. PMID: 19608138
-
Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia.Circulation. 2004 Sep 14;110(11):1398-405. doi: 10.1161/01.CIR.0000141563.71410.64. Epub 2004 Aug 30. Circulation. 2004. PMID: 15337699
-
Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium.Circ Res. 2011 Nov 11;109(11):1280-9. doi: 10.1161/CIRCRESAHA.111.248369. Epub 2011 Sep 29. Circ Res. 2011. PMID: 21959218 Free PMC article.
-
CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction.Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):11008-13. doi: 10.1073/pnas.0914248107. Epub 2010 Jun 1. Proc Natl Acad Sci U S A. 2010. PMID: 20534467 Free PMC article.
-
Pioneering therapies for post-infarction angiogenesis: Insight into molecular mechanisms and preclinical studies.Biomed Pharmacother. 2023 Oct;166:115306. doi: 10.1016/j.biopha.2023.115306. Epub 2023 Aug 10. Biomed Pharmacother. 2023. PMID: 37572633 Review.
Cited by
-
Sonic Hedgehog upregulation does not enhance the survival and engraftment of stem cell-derived cardiomyocytes in infarcted hearts.PLoS One. 2020 Jan 16;15(1):e0227780. doi: 10.1371/journal.pone.0227780. eCollection 2020. PLoS One. 2020. PMID: 31945113 Free PMC article.
-
Circulating fibrocyte mobilization in negative pressure wound therapy.J Cell Mol Med. 2017 Aug;21(8):1513-1522. doi: 10.1111/jcmm.13080. Epub 2017 Feb 17. J Cell Mol Med. 2017. PMID: 28211211 Free PMC article.
-
Alcohol consumption negates estrogen-mediated myocardial repair in ovariectomized mice by inhibiting endothelial progenitor cell mobilization and function.J Biol Chem. 2013 Jun 21;288(25):18022-34. doi: 10.1074/jbc.M113.468009. Epub 2013 May 3. J Biol Chem. 2013. PMID: 23645678 Free PMC article.
-
Enhancing endothelial progenitor cell for clinical use.World J Stem Cells. 2015 Jul 26;7(6):894-8. doi: 10.4252/wjsc.v7.i6.894. World J Stem Cells. 2015. PMID: 26240678 Free PMC article.
-
Gli1 promotes the phenotypic transformation of valve interstitial cells through Hedgehog pathway activation exacerbating calcific aortic valve disease.Int J Biol Sci. 2023 Apr 9;19(7):2053-2066. doi: 10.7150/ijbs.74123. eCollection 2023. Int J Biol Sci. 2023. PMID: 37151880 Free PMC article.
References
-
- Kawamoto A, Murayama T, Kusano K, et al. Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia. Circulation. 2004;110:1398–405. - PubMed
-
- Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27:1785–92. - PubMed
-
- Kang HJ, Kim HS, Zhang SY, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–6. - PubMed
-
- Kusano KF, Pola R, Murayama T, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–204. - PubMed
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL080137/HL/NHLBI NIH HHS/United States
- R01 HL057516/HL/NHLBI NIH HHS/United States
- HL-57516/HL/NHLBI NIH HHS/United States
- P01 HL066957/HL/NHLBI NIH HHS/United States
- R01 HL095874-01/HL/NHLBI NIH HHS/United States
- R01 HL080137-01A1/HL/NHLBI NIH HHS/United States
- HL-77428/HL/NHLBI NIH HHS/United States
- R37 HL053354/HL/NHLBI NIH HHS/United States
- R01 HL053354-08/HL/NHLBI NIH HHS/United States
- R01 HL053354-12/HL/NHLBI NIH HHS/United States
- R01 HL053354-11A2/HL/NHLBI NIH HHS/United States
- R01 HL057516-10/HL/NHLBI NIH HHS/United States
- R01 HL095874-03/HL/NHLBI NIH HHS/United States
- R01 HL080137-05/HL/NHLBI NIH HHS/United States
- R01 HL077428/HL/NHLBI NIH HHS/United States
- R01 HL095874-02/HL/NHLBI NIH HHS/United States
- R01 HL080137-03/HL/NHLBI NIH HHS/United States
- R01 HL053354-09/HL/NHLBI NIH HHS/United States
- HL-53354/HL/NHLBI NIH HHS/United States
- R01 HL053354-10/HL/NHLBI NIH HHS/United States
- R01 HL095874-04/HL/NHLBI NIH HHS/United States
- HL-80137/HL/NHLBI NIH HHS/United States
- R01 HL080137-02/HL/NHLBI NIH HHS/United States
- P01HL-66957/HL/NHLBI NIH HHS/United States
- R01 HL095874/HL/NHLBI NIH HHS/United States
- R01 HL057516-12/HL/NHLBI NIH HHS/United States
- R01 HL080137-04/HL/NHLBI NIH HHS/United States
- HL-95874/HL/NHLBI NIH HHS/United States
- R01 HL057516-11/HL/NHLBI NIH HHS/United States
- R01 HL057516-09/HL/NHLBI NIH HHS/United States
- R01 HL053354/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

