Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells
- PMID: 21656830
- PMCID: PMC3234312
- DOI: 10.1002/pros.21335
Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells
Abstract
Background: Nodal is a member of the transforming growth factor β (TGFβ) superfamily that directs embryonic patterning and promotes the plasticity and tumorigenicity of tumor cells, but its role in the prostate is unknown. The goal of this study was to characterize the expression and function of Nodal in prostate cancer and determine whether, like other TGFβ ligands, it modulates androgen receptor (AR) activity.
Methods: Nodal expression was investigated using immunohistochemistry of tissue microarrays and Western blots of prostate cell lines. The functional role of Nodal was examined using Matrigel and soft agar growth assays. Cross-talk between Nodal and AR signaling was assessed with luciferase reporter assays and expression of endogenous androgen regulated genes.
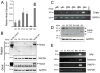
Results: Significantly increased Nodal expression was observed in cancer compared with benign prostate specimens. Nodal was only expressed by DU145 and PC3 cells. All cell lines expressed Nodal's co-receptor, Cripto-1, but lacked Lefty, a critical negative regulator of Nodal signaling. Recombinant human Nodal triggered downstream Smad2 phosphorylation in DU145 and LNCaP cells, and stable transfection of pre-pro-Nodal enhanced the growth of LNCaP cells in Matrigel and soft agar. Finally, Nodal attenuated AR signaling, reducing the activity of a PSA promoter construct in luciferase assays and down-regulating the endogenous expression of androgen regulated genes.
Conclusions: An aberrant Nodal signaling pathway is re-expressed and functionally active in prostate cancer cells.
Copyright © 2011 Wiley-Liss, Inc.
Figures

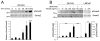

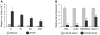
Similar articles
-
Differential role of Sloan-Kettering Institute (Ski) protein in Nodal and transforming growth factor-beta (TGF-β)-induced Smad signaling in prostate cancer cells.Carcinogenesis. 2012 Nov;33(11):2054-64. doi: 10.1093/carcin/bgs252. Epub 2012 Jul 27. Carcinogenesis. 2012. PMID: 22843506 Free PMC article.
-
Expression of nodal and nodal receptors in prostate stem cells and prostate cancer cells: autocrine effects on cell proliferation and migration.Prostate. 2011 Jul;71(10):1084-96. doi: 10.1002/pros.21326. Epub 2011 Jan 12. Prostate. 2011. PMID: 21557273 Free PMC article.
-
Transcriptional regulation of the androgen signaling pathway by the Wilms' tumor suppressor gene WT1.Anticancer Res. 2001 Jan-Feb;21(1A):1-10. Anticancer Res. 2001. PMID: 11299720
-
Interleukin-6 regulates androgen receptor activity and prostate cancer cell growth.Mol Cell Endocrinol. 2002 Nov 29;197(1-2):231-8. doi: 10.1016/s0303-7207(02)00263-0. Mol Cell Endocrinol. 2002. PMID: 12431817 Review.
-
Spatial and temporal control of NODAL signaling.Curr Opin Cell Biol. 2018 Apr;51:50-57. doi: 10.1016/j.ceb.2017.10.005. Epub 2017 Nov 15. Curr Opin Cell Biol. 2018. PMID: 29153705 Review.
Cited by
-
Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance.Int J Med Sci. 2015 Jan 1;12(1):63-71. doi: 10.7150/ijms.9982. eCollection 2015. Int J Med Sci. 2015. PMID: 25552920 Free PMC article.
-
Back to the embryonic stage: Nodal as a biomarker for breast cancer progression.Breast Cancer Res. 2012 May 21;14(3):105. doi: 10.1186/bcr3177. Breast Cancer Res. 2012. PMID: 22643182 Free PMC article.
-
ACVR1C/SMAD2 signaling promotes invasion and growth in retinoblastoma.Oncogene. 2019 Mar;38(12):2056-2075. doi: 10.1038/s41388-018-0543-2. Epub 2018 Nov 6. Oncogene. 2019. PMID: 30401983 Free PMC article.
-
Gefitinib and luteolin cause growth arrest of human prostate cancer PC-3 cells via inhibition of cyclin G-associated kinase and induction of miR-630.PLoS One. 2014 Jun 27;9(6):e100124. doi: 10.1371/journal.pone.0100124. eCollection 2014. PLoS One. 2014. PMID: 24971999 Free PMC article.
-
Melanoma tumor cell heterogeneity: a molecular approach to study subpopulations expressing the embryonic morphogen nodal.Semin Oncol. 2014 Apr;41(2):259-266. doi: 10.1053/j.seminoncol.2014.02.001. Epub 2014 Feb 7. Semin Oncol. 2014. PMID: 24787297 Free PMC article. Review.
References
-
- Pierce GB, Speers WC. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 1988;48(8):1996–2004. - PubMed
-
- Marker PC. Does prostate cancer co-opt the developmental program? Differentiation. 2008;76(6):736–744. - PubMed
-
- Pritchard C, Mecham B, Dumpit R, Coleman I, Bhattacharjee M, Chen Q, Sikes RA, Nelson PS. Conserved gene expression programs integrate mammalian prostate development and tumorigenesis. Cancer Res. 2009;69(5):1739–1747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

