Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis
- PMID: 21646721
- PMCID: PMC3223840
- DOI: 10.1172/JCI57275
Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis
Abstract
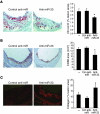
Plasma HDL levels have a protective role in atherosclerosis, yet clinical therapies to raise HDL levels have remained elusive. Recent advances in the understanding of lipid metabolism have revealed that miR-33, an intronic microRNA located within the SREBF2 gene, suppresses expression of the cholesterol transporter ABC transporter A1 (ABCA1) and lowers HDL levels. Conversely, mechanisms that inhibit miR-33 increase ABCA1 and circulating HDL levels, suggesting that antagonism of miR-33 may be atheroprotective. As the regression of atherosclerosis is clinically desirable, we assessed the impact of miR-33 inhibition in mice deficient for the LDL receptor (Ldlr-/- mice), with established atherosclerotic plaques. Mice treated with anti-miR33 for 4 weeks showed an increase in circulating HDL levels and enhanced reverse cholesterol transport to the plasma, liver, and feces. Consistent with this, anti-miR33-treated mice showed reductions in plaque size and lipid content, increased markers of plaque stability, and decreased inflammatory gene expression. Notably, in addition to raising ABCA1 levels in the liver, anti-miR33 oligonucleotides directly targeted the plaque macrophages, in which they enhanced ABCA1 expression and cholesterol removal. These studies establish that raising HDL levels by anti-miR33 oligonucleotide treatment promotes reverse cholesterol transport and atherosclerosis regression and suggest that it may be a promising strategy to treat atherosclerotic vascular disease.
Figures
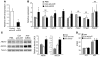

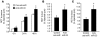

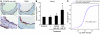
Similar articles
-
miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice.Circ Res. 2014 Oct 10;115(9):759-69. doi: 10.1161/CIRCRESAHA.115.304164. Epub 2014 Sep 8. Circ Res. 2014. PMID: 25201910 Free PMC article.
-
microRNAs in lipoprotein metabolism and cardiometabolic disorders.Atherosclerosis. 2016 Mar;246:352-60. doi: 10.1016/j.atherosclerosis.2016.01.025. Epub 2016 Jan 18. Atherosclerosis. 2016. PMID: 26828754 Free PMC article. Review.
-
Inhibition of soluble epoxide hydrolase in mice promotes reverse cholesterol transport and regression of atherosclerosis.Atherosclerosis. 2015 Apr;239(2):557-65. doi: 10.1016/j.atherosclerosis.2015.02.014. Epub 2015 Feb 16. Atherosclerosis. 2015. PMID: 25733327 Free PMC article.
-
MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):323-31. doi: 10.1161/ATVBAHA.114.304878. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524771 Free PMC article.
-
[Role of the ABC transporters A1 and G1, key reverse cholesterol transport proteins, in atherosclerosis].Mol Biol (Mosk). 2016 Mar-Apr;50(2):223-30. doi: 10.7868/S002689841602004X. Mol Biol (Mosk). 2016. PMID: 27239842 Review. Russian.
Cited by
-
The plaque "micro" environment: microRNAs control the risk and the development of atherosclerosis.Curr Atheroscler Rep. 2012 Oct;14(5):413-21. doi: 10.1007/s11883-012-0272-x. Curr Atheroscler Rep. 2012. PMID: 22847770 Free PMC article. Review.
-
Studies in a Murine Granuloma Model of Instilled Carbon Nanotubes: Relevance to Sarcoidosis.Int J Mol Sci. 2021 Apr 2;22(7):3705. doi: 10.3390/ijms22073705. Int J Mol Sci. 2021. PMID: 33918196 Free PMC article. Review.
-
MicroRNAs in Liver Health and Disease.Curr Pathobiol Rep. 2013 Mar;1(1):53-62. doi: 10.1007/s40139-012-0005-4. Curr Pathobiol Rep. 2013. PMID: 23565350 Free PMC article.
-
Association Study Between Metabolic Syndrome and rs8066560 Polymorphism in the Promoter Region of Sterol Regulatory Element-binding Transcription Factor 1 Gene in Iranian Children and Adolescents.Int J Prev Med. 2016 Feb 23;7:41. doi: 10.4103/2008-7802.177314. eCollection 2016. Int J Prev Med. 2016. PMID: 27076879 Free PMC article.
-
Resveratrol suppresses hepatic fatty acid synthesis and increases fatty acid β-oxidation via the microRNA-33/SIRT6 signaling pathway.Exp Ther Med. 2024 Jun 19;28(2):326. doi: 10.3892/etm.2024.12615. eCollection 2024 Aug. Exp Ther Med. 2024. PMID: 38979023 Free PMC article.
References
-
- Castelli WP, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55(5):767–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL084312/HL/NHLBI NIH HHS/United States
- R01 HL107953/HL/NHLBI NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01AG020255/AG/NIA NIH HHS/United States
- R00 HL088528/HL/NHLBI NIH HHS/United States
- R01 AG020255/AG/NIA NIH HHS/United States
- R01 HL084312/HL/NHLBI NIH HHS/United States
- R01HL108182/HL/NHLBI NIH HHS/United States
- K99 HL088528/HL/NHLBI NIH HHS/United States
- R01 HL108182/HL/NHLBI NIH HHS/United States
- P01HL098055/HL/NHLBI NIH HHS/United States
- P01 HL098055/HL/NHLBI NIH HHS/United States
- R01HL107953/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

