Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial
- PMID: 21641636
- PMCID: PMC3159415
- DOI: 10.1016/S0140-6736(11)60613-2
Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial
Abstract
Background: In the Medical Research Council (MRC) COIN trial, the epidermal growth factor receptor (EGFR)-targeted antibody cetuximab was added to standard chemotherapy in first-line treatment of advanced colorectal cancer with the aim of assessing effect on overall survival.
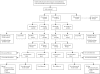
Methods: In this randomised controlled trial, patients who were fit for but had not received previous chemotherapy for advanced colorectal cancer were randomly assigned to oxaliplatin and fluoropyrimidine chemotherapy (arm A), the same combination plus cetuximab (arm B), or intermittent chemotherapy (arm C). The choice of fluoropyrimidine therapy (capecitabine or infused fluouroracil plus leucovorin) was decided before randomisation. Randomisation was done centrally (via telephone) by the MRC Clinical Trials Unit using minimisation. Treatment allocation was not masked. The comparison of arms A and C is described in a companion paper. Here, we present the comparison of arm A and B, for which the primary outcome was overall survival in patients with KRAS wild-type tumours. Analysis was by intention to treat. Further analyses with respect to NRAS, BRAF, and EGFR status were done. The trial is registered, ISRCTN27286448.
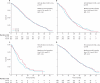
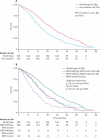
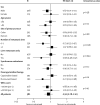
Findings: 1630 patients were randomly assigned to treatment groups (815 to standard therapy and 815 to addition of cetuximab). Tumour samples from 1316 (81%) patients were used for somatic molecular analyses; 565 (43%) had KRAS mutations. In patients with KRAS wild-type tumours (arm A, n=367; arm B, n=362), overall survival did not differ between treatment groups (median survival 17·9 months [IQR 10·3-29·2] in the control group vs 17·0 months [9·4-30·1] in the cetuximab group; HR 1·04, 95% CI 0·87-1·23, p=0·67). Similarly, there was no effect on progression-free survival (8·6 months [IQR 5·0-12·5] in the control group vs 8·6 months [5·1-13·8] in the cetuximab group; HR 0·96, 0·82-1·12, p=0·60). Overall response rate increased from 57% (n=209) with chemotherapy alone to 64% (n=232) with addition of cetuximab (p=0·049). Grade 3 and higher skin and gastrointestinal toxic effects were increased with cetuximab (14 vs 114 and 67 vs 97 patients in the control group vs the cetuximab group with KRAS wild-type tumours, respectively). Overall survival differs by somatic mutation status irrespective of treatment received: BRAF mutant, 8·8 months (IQR 4·5-27·4); KRAS mutant, 14·4 months (8·5-24·0); all wild-type, 20·1 months (11·5-31·7).
Interpretation: This trial has not confirmed a benefit of addition of cetuximab to oxaliplatin-based chemotherapy in first-line treatment of patients with advanced colorectal cancer. Cetuximab increases response rate, with no evidence of benefit in progression-free or overall survival in KRAS wild-type patients or even in patients selected by additional mutational analysis of their tumours. The use of cetuximab in combination with oxaliplatin and capecitabine in first-line chemotherapy in patients with widespread metastases cannot be recommended.
Funding: Cancer Research UK, Cancer Research Wales, UK Medical Research Council, Merck KGgA.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
First-line treatment of advanced colorectal cancer.Lancet. 2011 Jun 18;377(9783):2060-2. doi: 10.1016/S0140-6736(11)60788-5. Epub 2011 Jun 5. Lancet. 2011. PMID: 21641637 No abstract available.
Similar articles
-
Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial.Lancet Oncol. 2014 May;15(6):631-9. doi: 10.1016/S1470-2045(14)70106-8. Epub 2014 Apr 3. Lancet Oncol. 2014. PMID: 24703531 Free PMC article. Clinical Trial.
-
Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer.N Engl J Med. 2009 Feb 5;360(6):563-72. doi: 10.1056/NEJMoa0808268. N Engl J Med. 2009. PMID: 19196673 Clinical Trial.
-
Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104--a randomized trial of the German AIO CRC study group.J Clin Oncol. 2011 Mar 10;29(8):1050-8. doi: 10.1200/JCO.2010.31.1936. Epub 2011 Feb 7. J Clin Oncol. 2011. PMID: 21300933 Clinical Trial.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome.Clin Transl Oncol. 2010 Aug;12(8):533-42. doi: 10.1007/s12094-010-0551-3. Clin Transl Oncol. 2010. PMID: 20709651 Review.
Cited by
-
Evaluation in usual practice of the bevacizumab-FOLFIRI combination for the first-line treatment of patients with unresectable metastatic colorectal cancer treated in 2006: focus on resected patients and oncogeriatrics: AVASTIN OUEST cohort of the Observatory of Cancer of the Brittany and Pays de la Loire Areas (Observatoire dédié au Cancer Bretagne / Pays de la Loire).Oncologie (Paris). 2014;16(5):267-276. doi: 10.1007/s10269-014-2391-1. Epub 2014 Dec 21. Oncologie (Paris). 2014. PMID: 26190928 Free PMC article.
-
An update on chemotherapy of colorectal liver metastases.World J Gastroenterol. 2012 Jan 7;18(1):25-33. doi: 10.3748/wjg.v18.i1.25. World J Gastroenterol. 2012. PMID: 22228967 Free PMC article. Review.
-
First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study.Oncologist. 2012;17(3):339-45. doi: 10.1634/theoncologist.2011-0406. Epub 2012 Feb 23. Oncologist. 2012. PMID: 22363067 Free PMC article. Clinical Trial.
-
First-line chemotherapy for mCRC—a review and evidence-based algorithm.Nat Rev Clin Oncol. 2015 Oct;12(10):607-19. doi: 10.1038/nrclinonc.2015.129. Epub 2015 Jul 28. Nat Rev Clin Oncol. 2015. PMID: 26215044 Review.
-
KRAS and BRAF gene mutations and DNA mismatch repair status in Chinese colorectal carcinoma patients.World J Gastroenterol. 2015 Feb 7;21(5):1595-605. doi: 10.3748/wjg.v21.i5.1595. World J Gastroenterol. 2015. PMID: 25663779 Free PMC article.
References
-
- Lynch TJ, Bell DW, Sordella R. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
-
- Paez JG, Jänne PA, Lee JC. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
-
- Khambata-Ford S, Garrett CR, Meropol NJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. - PubMed
-
- Lièvre A, Bachet JB, Le Corre D. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. - PubMed
-
- Karapetis CS, Khambata-Ford S, Jonker DJ. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

