Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy
- PMID: 21635241
- PMCID: PMC3169746
- DOI: 10.1111/j.1528-1167.2011.03115.x
Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy
Abstract
Purpose: Adenosine kinase (ADK) represents the key metabolic enzyme for the regulation of extracellular adenosine levels in the brain. In adult brain, ADK is primarily present in astrocytes. Several lines of experimental evidence support a critical role of ADK in different types of brain injury associated with astrogliosis, which is also a prominent morphologic feature of temporal lobe epilepsy (TLE). We hypothesized that dysregulation of ADK is an ubiquitous pathologic hallmark of TLE.
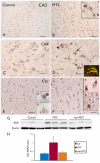
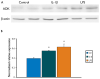
Methods: Using immunocytochemistry and Western blot analysis, we investigated ADK protein expression in a rat model of TLE during epileptogenesis and the chronic epileptic phase and compared those findings with tissue resected from TLE patients with mesial temporal sclerosis (MTS).
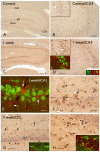
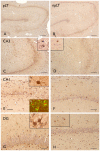
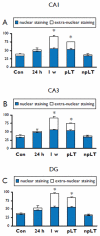
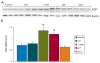
Key findings: In rat control hippocampus and cortex, a low baseline expression of ADK was found with mainly nuclear localization. One week after the electrical induction of status epilepticus (SE), prominent up-regulation of ADK became evident in astrocytes with a characteristic cytoplasmic localization. This increase in ADK persisted at least for 3-4 months after SE in rats developing a progressive form of epilepsy. In line with the findings from the rat model, expression of astrocytic ADK was also found to be increased in the hippocampus and temporal cortex of patients with TLE. In addition, in vitro experiments in human astrocyte cultures showed that ADK expression was increased by several proinflammatory molecules (interleukin-1β and lipopolysaccharide).
Significance: These results suggest that dysregulation of ADK in astrocytes is a common pathologic hallmark of TLE. Moreover, in vitro data suggest the existence of an additional layer of modulatory crosstalk between the astrocyte-based adenosine cycle and inflammation. Whether this interaction also can play a role in vivo needs to be further investigated.
Wiley Periodicals, Inc. © 2011 International League Against Epilepsy.
Figures
Similar articles
-
Increased expression of (immuno)proteasome subunits during epileptogenesis is attenuated by inhibition of the mammalian target of rapamycin pathway.Epilepsia. 2017 Aug;58(8):1462-1472. doi: 10.1111/epi.13823. Epub 2017 Jun 23. Epilepsia. 2017. PMID: 28643873
-
Dynamic Changes of Astrocytes and Adenosine Signaling in Rat Hippocampus in Post-status Epilepticus Model of Epileptogenesis.Cell Mol Neurobiol. 2018 Aug;38(6):1227-1234. doi: 10.1007/s10571-018-0590-9. Epub 2018 May 16. Cell Mol Neurobiol. 2018. PMID: 29770956
-
Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation.Brain. 2005 Oct;128(Pt 10):2383-95. doi: 10.1093/brain/awh555. Epub 2005 Jun 1. Brain. 2005. PMID: 15930047
-
The adenosine kinase hypothesis of epileptogenesis.Prog Neurobiol. 2008 Mar;84(3):249-62. doi: 10.1016/j.pneurobio.2007.12.002. Epub 2007 Dec 23. Prog Neurobiol. 2008. PMID: 18249058 Free PMC article. Review.
-
Adenosine dysfunction in epilepsy.Glia. 2012 Aug;60(8):1234-43. doi: 10.1002/glia.22285. Epub 2011 Dec 22. Glia. 2012. PMID: 22700220 Free PMC article. Review.
Cited by
-
Acetate supplementation modulates brain adenosine metabolizing enzymes and adenosine A₂A receptor levels in rats subjected to neuroinflammation.J Neuroinflammation. 2014 Jun 4;11:99. doi: 10.1186/1742-2094-11-99. J Neuroinflammation. 2014. PMID: 24898794 Free PMC article.
-
Astrocytes and Epilepsy.Neurochem Res. 2021 Oct;46(10):2687-2695. doi: 10.1007/s11064-021-03236-x. Epub 2021 Mar 4. Neurochem Res. 2021. PMID: 33661442
-
Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats.Neuropharmacology. 2015 Dec;99:500-9. doi: 10.1016/j.neuropharm.2015.08.007. Epub 2015 Aug 6. Neuropharmacology. 2015. PMID: 26256422 Free PMC article.
-
General Pathophysiology of Astroglia.Adv Exp Med Biol. 2019;1175:149-179. doi: 10.1007/978-981-13-9913-8_7. Adv Exp Med Biol. 2019. PMID: 31583588 Free PMC article. Review.
-
The Interaction Between Sleep and Epilepsy.Curr Neurol Neurosci Rep. 2022 Sep;22(9):551-563. doi: 10.1007/s11910-022-01219-1. Epub 2022 Jul 8. Curr Neurol Neurosci Rep. 2022. PMID: 35802300 Review.
References
-
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:2106–2118. - PubMed
-
- Aronica E, Gorter JA, Rozemuller AJ, Yankaya B, Troost D. Interleukin-1 beta down-regulates the expression of metabotropic glutamate receptor 5 in cultured human astrocytes. J Neuroimmunol. 2005;160:188–194. - PubMed
-
- Aronica E, Gorter J. Gene Expression Profile in Temporal Lobe Epilepsy. Neuroscientist. 2007;13:1–9. - PubMed
-
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. GLIA. 2006;54:358–368. - PubMed
-
- Blumcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstock J, Stefan H, Hildebrandt M. A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol. 2007;113:235–244. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

