Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis
- PMID: 21630268
- PMCID: PMC3236253
- DOI: 10.1002/ijc.26217
Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis
Abstract
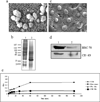
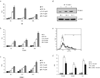
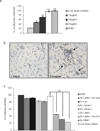
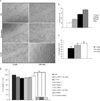
Our study is designed to assess if exosomes released from chronic myelogenous leukemia (CML) cells may modulate angiogenesis. We have isolated and characterized the exosomes generated from LAMA84 CML cells and demonstrated that addition of exosomes to human vascular endothelial cells (HUVEC) induces an increase of both ICAM-1 and VCAM-1 cell adhesion molecules and interleukin-8 expression. The stimulation of cell-cell adhesion molecules was paralleled by a dose-dependent increase of adhesion of CML cells to a HUVEC monolayer. We further showed that the treatment with exosomes from CML cells caused an increase in endothelial cell motility accompanied by a loss of VE-cadherin and β-catenin from the endothelial cell surface. Functional characterization of exosomes isolated from CML patients confirmed the data obtained with exosomes derived from CML cell line. CML exosomes caused reorganization into tubes of HUVEC cells cultured on Matrigel. When added to Matrigel plugs in vivo, exosomes induced ingrowth of murine endothelial cells and vascularization of the Matrigel plugs. Our results suggest for the first time that exosomes released from CML cells directly affect endothelial cells modulating the process of neovascularization.
Copyright © 2011 UICC.
Figures

Similar articles
-
Carboxyamidotriazole-orotate inhibits the growth of imatinib-resistant chronic myeloid leukaemia cells and modulates exosomes-stimulated angiogenesis.PLoS One. 2012;7(8):e42310. doi: 10.1371/journal.pone.0042310. Epub 2012 Aug 3. PLoS One. 2012. Retraction in: PLoS One. 2019 Oct 16;14(10):e0224237. doi: 10.1371/journal.pone.0224237. PMID: 22879938 Free PMC article. Retracted.
-
Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion.Angiogenesis. 2012 Mar;15(1):33-45. doi: 10.1007/s10456-011-9241-1. Epub 2011 Dec 22. Angiogenesis. 2012. PMID: 22203239 Free PMC article.
-
Exosomal shuttling of miR-126 in endothelial cells modulates adhesive and migratory abilities of chronic myelogenous leukemia cells.Mol Cancer. 2014 Jul 11;13:169. doi: 10.1186/1476-4598-13-169. Mol Cancer. 2014. PMID: 25015105 Free PMC article.
-
Crosstalk between CML cells with HUVECS and BMSCs through CML derived exosomes.Front Biosci (Landmark Ed). 2021 Jan 1;26(3):444-467. doi: 10.2741/4901. Front Biosci (Landmark Ed). 2021. PMID: 33049677 Review.
-
[Endothelial cell adhesion molecules].Usp Fiziol Nauk. 2014 Oct-Dec;45(4):34-49. Usp Fiziol Nauk. 2014. PMID: 25729843 Review. Russian.
Cited by
-
Carboxyamidotriazole-orotate inhibits the growth of imatinib-resistant chronic myeloid leukaemia cells and modulates exosomes-stimulated angiogenesis.PLoS One. 2012;7(8):e42310. doi: 10.1371/journal.pone.0042310. Epub 2012 Aug 3. PLoS One. 2012. Retraction in: PLoS One. 2019 Oct 16;14(10):e0224237. doi: 10.1371/journal.pone.0224237. PMID: 22879938 Free PMC article. Retracted.
-
Extracellular Vesicles in Haematological Disorders: A Friend or a Foe?Int J Mol Sci. 2022 Sep 4;23(17):10118. doi: 10.3390/ijms231710118. Int J Mol Sci. 2022. PMID: 36077514 Free PMC article. Review.
-
The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions.Biomed Res Int. 2014;2014:693157. doi: 10.1155/2014/693157. Epub 2014 Sep 14. Biomed Res Int. 2014. PMID: 25302305 Free PMC article. Review.
-
VE-cadherin cleavage by ovarian cancer microparticles induces β-catenin phosphorylation in endothelial cells.Oncotarget. 2016 Feb 2;7(5):5289-305. doi: 10.18632/oncotarget.6677. Oncotarget. 2016. PMID: 26700621 Free PMC article.
-
Exosome Secretion - More Than Simple Waste Disposal? Implications for Physiology, Diagnostics and Therapeutics.J Circ Biomark. 2016 Apr 1;5:7. doi: 10.5772/62975. eCollection 2016 Jan-Dec. J Circ Biomark. 2016. PMID: 28936255 Free PMC article. Review.
References
-
- Rowley J. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. - PubMed
-
- Jabbour E, Cortes JE, Ghanem H, O'Brien S, Kantarjian HM. Targeted therapy in chronic myeloid leukemia. Expert Rev Anticancer Ther. 2008;8:99–110. - PubMed
-
- Legros L, Bourcier C, Jacquel A, Mahon FX, Cassuto JP, Auberger P, Pages G. Imatinib mesylate (STI571) decreases the vascular endothelial growth factor plasma concentration in patients with chronic myeloid leukemia. Blood. 2004;104:495–501. - PubMed
-
- Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O'Brien S, Keating M, Freireich E, Albitar M. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–2245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

