S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata
- PMID: 21622839
- PMCID: PMC3170554
- DOI: 10.1093/jxb/err171
S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata
Abstract
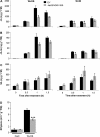
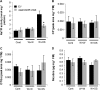
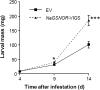
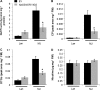
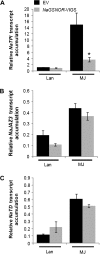
S-nitrosoglutathione reductase (GSNOR) reduces the nitric oxide (NO) adduct S-nitrosoglutathione (GSNO), an essential reservoir for NO bioactivity. In plants, GSNOR has been found to be important in resistance to bacterial and fungal pathogens, but whether it is also involved in plant-herbivore interactions was not known. Using a virus-induced gene silencing (VIGS) system, the activity of GSNOR in a wild tobacco species, Nicotiana attenuata, was knocked down and the function of GSNOR in defence against the insect herbivore Manduca sexta was examined. Silencing GSNOR decreased the herbivory-induced accumulation of jasmonic acid (JA) and ethylene, two important phytohormones regulating plant defence levels, without compromising the activity of two mitogen-activated protein kinases (MAPKs), salicylic acid-induced protein kinase (SIPK) and wound-induced protein kinase (WIPK). Decreased activity of trypsin proteinase inhibitors (TPIs) were detected in GSNOR-silenced plants after simulated M. sexta feeding and bioassays indicated that GSNOR-silenced plants have elevated susceptibility to M. sexta attack. Furthermore, GSNOR is required for methyl jasmonate (MeJA)-induced accumulation of defence-related secondary metabolites (TPI, caffeoylputrescine, and diterpene glycosides) but is not needed for the transcriptional regulation of JAZ3 (jasmonate ZIM-domain 3) and TD (threonine deaminase), indicating that GSNOR mediates certain but not all jasmonate-inducible responses. This work highlights the important role of GSNOR in plant resistance to herbivory and jasmonate signalling and suggests the potential involvement of NO in plant-herbivore interactions. Our data also suggest that GSNOR could be a target of genetic modification for improving crop resistance to herbivores.
© 2011 The Author(s).
Figures
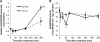
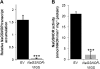

Similar articles
-
Two mitogen-activated protein kinase kinases, MKK1 and MEK2, are involved in wounding- and specialist lepidopteran herbivore Manduca sexta-induced responses in Nicotiana attenuata.J Exp Bot. 2011 Aug;62(12):4355-65. doi: 10.1093/jxb/err162. Epub 2011 May 24. J Exp Bot. 2011. PMID: 21610019 Free PMC article.
-
Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly up-regulates wound- and herbivory-induced jasmonic acid accumulations.Plant Physiol. 2012 Aug;159(4):1591-607. doi: 10.1104/pp.112.199018. Epub 2012 Jun 19. Plant Physiol. 2012. PMID: 22715110 Free PMC article.
-
Ethylene modulates the role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in cross talk between salicylate and jasmonate signaling.Plant Physiol. 2009 Apr;149(4):1797-809. doi: 10.1104/pp.108.133926. Epub 2009 Jan 28. Plant Physiol. 2009. PMID: 19176718 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
Cited by
-
GSNOR Contributes to Demethylation and Expression of Transposable Elements and Stress-Responsive Genes.Antioxidants (Basel). 2021 Jul 15;10(7):1128. doi: 10.3390/antiox10071128. Antioxidants (Basel). 2021. PMID: 34356361 Free PMC article.
-
Arabidopsis redox status in response to caterpillar herbivory.Front Plant Sci. 2013 May 6;4:113. doi: 10.3389/fpls.2013.00113. eCollection 2013. Front Plant Sci. 2013. PMID: 23653629 Free PMC article.
-
S-Nitrosoglutathione Reductase-The Master Regulator of Protein S-Nitrosation in Plant NO Signaling.Plants (Basel). 2019 Feb 21;8(2):48. doi: 10.3390/plants8020048. Plants (Basel). 2019. PMID: 30795534 Free PMC article. Review.
-
Hemoglobin regulation of plant embryogenesis and plant pathogen interaction.Plant Signal Behav. 2013 Aug;8(8):e25264. doi: 10.4161/psb.25264. Epub 2013 Jun 7. Plant Signal Behav. 2013. PMID: 23759548 Free PMC article. Review.
-
Arabidopsis Transcriptomics Reveals the Role of Lipoxygenase2 (AtLOX2) in Wound-Induced Responses.Int J Mol Sci. 2024 May 28;25(11):5898. doi: 10.3390/ijms25115898. Int J Mol Sci. 2024. PMID: 38892085 Free PMC article.
References
-
- Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annual Review of Plant Biology. 2008;59:21–39. - PubMed
-
- Chen M-S. Inducible direct plant defense against insect herbivores: a review. Insect Science. 2008;15:101–114. - PubMed
-
- Chini A, Fonseca S, Fernandez G, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

