The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model
- PMID: 21613216
- PMCID: PMC3143630
- DOI: 10.1074/jbc.M110.193987
The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model
Abstract
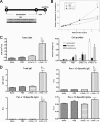
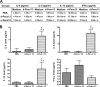
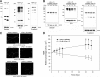
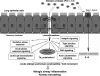
Fungal allergens are associated with the development of asthma, and some have been characterized as proteases. Here, we established an animal model of allergic airway inflammation in response to continuous exposure to proteolytically active Pen c 13, a major allergen secreted by Penicillium citrinum. In functional analyses, Pen c 13 exposure led to increased airway hyperresponsiveness, significant inflammatory cell infiltration, mucus overproduction, and collagen deposition in the lung, dramatically elevated serum levels of total IgE and Pen c 13-specific IgE and IgG1, and increased production of the Th2 cytokines IL-4, IL-5, and IL-13 by splenocytes stimulated in vitro with Pen c 13. To examine the mechanisms involved in the regulation of allergenicity by Pen c 13, we performed two-dimensional fluorescence difference gel electrophoresis analysis combined with nano-LC-MS/MS, followed by bioinformatics analysis to identify potential targets that associated with allergic inflammation, which suggested that galectin-3 and laminin might be involved in novel pathogenic mechanisms. Finally, we focused on junctional proteins between cells, because, in addition to opening of the epithelial barrier by environmental proteases possibly being the initial step in the development of asthma, these proteins are also associated with actin rearrangement. Taken together, our findings indicate that Pen c 13 exposure causes junctional structure alterations and actin cytoskeletal rearrangements, resulting in increased permeability and airway structural changes. These effects probably change the lung microenvironment and foster the development of allergic sensitization.
Figures



Similar articles
-
Identification of critical amino acids in an immunodominant IgE epitope of Pen c 13, a major allergen from Penicillium citrinum.PLoS One. 2012;7(4):e34627. doi: 10.1371/journal.pone.0034627. Epub 2012 Apr 10. PLoS One. 2012. PMID: 22506037 Free PMC article.
-
Aspergillus fumigatus proteases, Asp f 5 and Asp f 13, are essential for airway inflammation and remodelling in a murine inhalation model.Clin Exp Allergy. 2015 May;45(5):982-993. doi: 10.1111/cea.12426. Clin Exp Allergy. 2015. PMID: 25270353
-
Role of SHIP-1 in the adaptive immune responses to aeroallergen in the airway.PLoS One. 2010 Nov 30;5(11):e14174. doi: 10.1371/journal.pone.0014174. PLoS One. 2010. PMID: 21151496 Free PMC article.
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
Potential of immunoglobulin A to prevent allergic asthma.Clin Dev Immunol. 2013;2013:542091. doi: 10.1155/2013/542091. Epub 2013 Apr 11. Clin Dev Immunol. 2013. PMID: 23690823 Free PMC article. Review.
Cited by
-
Pathogenesis, Diagnosis, and Treatment of Infectious Rhinosinusitis.Microorganisms. 2024 Aug 16;12(8):1690. doi: 10.3390/microorganisms12081690. Microorganisms. 2024. PMID: 39203531 Free PMC article. Review.
-
Initiation and Pathogenesis of Severe Asthma with Fungal Sensitization.Cells. 2021 Apr 15;10(4):913. doi: 10.3390/cells10040913. Cells. 2021. PMID: 33921169 Free PMC article. Review.
-
Sensing of protease activity as a triggering mechanism of Th2 cell immunity and allergic disease.Front Allergy. 2023 Sep 21;4:1265049. doi: 10.3389/falgy.2023.1265049. eCollection 2023. Front Allergy. 2023. PMID: 37810200 Free PMC article. Review.
-
Protease allergens as initiators-regulators of allergic inflammation.Allergy. 2023 May;78(5):1148-1168. doi: 10.1111/all.15678. Epub 2023 Feb 26. Allergy. 2023. PMID: 36794967 Free PMC article. Review.
-
The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources.World Allergy Organ J. 2020 Apr 29;13(5):100118. doi: 10.1016/j.waojou.2020.100118. eCollection 2020 May. World Allergy Organ J. 2020. PMID: 32373267 Free PMC article. Review.
References
-
- Lai C. K., De Guia T. S., Kim Y. Y., Kuo S. H., Mukhopadhyay A., Soriano J. B., Trung P. L., Zhong N. S., Zainudin N., Zainudin B. M. (2003) J. Allergy Clin. Immunol. 111, 263–268 - PubMed
-
- Rabe K. F., Adachi M., Lai C. K., Soriano J. B., Vermeire P. A., Weiss K. B., Weiss S. T. (2004) J. Allergy Clin. Immunol. 114, 40–47 - PubMed
-
- Gould H. J., Sutton B. J. (2008) Nat. Rev. Immunol. 8, 205–217 - PubMed
-
- Folli C., Descalzi D., Scordamaglia F., Riccio A. M., Gamalero C., Canonica G. W. (2008) Curr. Opin. Allergy Clin. Immunol. 8, 367–375 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

