Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis
- PMID: 21610693
- PMCID: PMC3155296
- DOI: 10.1038/emboj.2011.159
Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis
Abstract
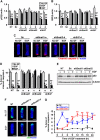
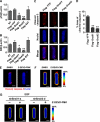
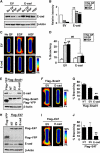
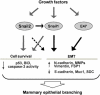
Several E-box-binding transcription factors regulate individual and collective cell migration and enhance the motility of epithelial cells by promoting epithelial-mesenchymal transition (EMT). Here, we characterized the role of a subset of these transcription factors and the EMT proteome in branching morphogenesis of mammary epithelial tissues using a three-dimensional organotypic culture model of the mammary duct. We found that the transcription factors Snail1, Snail2, and E47 were transiently upregulated at branch sites; decreasing the expression of these transcription factors inhibited branching. Conversely, ectopic expression of Snail1, Snail2, and E47 induced branching in the absence of exogenous stimuli. These changes correlated with the expression of mesenchymal markers and repression of E-cadherin, which was essential for branching. Snail1 and Snail2 also promoted cell survival at branch sites, but this was not sufficient to induce branching. These findings indicate that Snail1, Snail2, and E47 can promote collective migration during branching morphogenesis of mammary epithelial tissues through key regulators of EMT.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
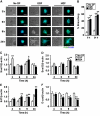
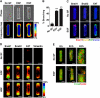
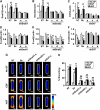
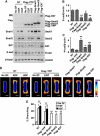
Similar articles
-
Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition.J Biol Chem. 2014 Jan 10;289(2):930-41. doi: 10.1074/jbc.M113.528026. Epub 2013 Dec 1. J Biol Chem. 2014. PMID: 24297167 Free PMC article.
-
Prep1 (pKnox1) transcription factor contributes to pubertal mammary gland branching morphogenesis.Int J Dev Biol. 2018;62(11-12):827-836. doi: 10.1387/ijdb.180278fb. Int J Dev Biol. 2018. PMID: 30604852
-
HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition.J Biol Chem. 2008 Nov 28;283(48):33437-46. doi: 10.1074/jbc.M802016200. Epub 2008 Oct 1. J Biol Chem. 2008. PMID: 18832382 Free PMC article.
-
Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention.J Exp Clin Cancer Res. 2014 Aug 2;33(1):62. doi: 10.1186/s13046-014-0062-0. J Exp Clin Cancer Res. 2014. PMID: 25084828 Free PMC article. Review.
-
Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer.Am J Pathol. 2012 Jun;180(6):2188-200. doi: 10.1016/j.ajpath.2012.02.031. Epub 2012 Apr 26. Am J Pathol. 2012. PMID: 22542493 Free PMC article. Review.
Cited by
-
Snail-induced epithelial-to-mesenchymal transition of MCF-7 breast cancer cells: systems analysis of molecular changes and their effect on radiation and drug sensitivity.BMC Cancer. 2016 Mar 18;16:236. doi: 10.1186/s12885-016-2274-5. BMC Cancer. 2016. PMID: 26988558 Free PMC article.
-
Paracrine Met signaling triggers epithelial-mesenchymal transition in mammary luminal progenitors, affecting their fate.Elife. 2015 Jul 13;4:e06104. doi: 10.7554/eLife.06104. Elife. 2015. PMID: 26165517 Free PMC article.
-
Regulation of Epithelial-Mesenchymal Transition by Transmission of Mechanical Stress through Epithelial Tissues.Cancer Microenviron. 2012 Apr;5(1):29-38. doi: 10.1007/s12307-011-0076-5. Epub 2011 Jul 12. Cancer Microenviron. 2012. PMID: 21748438 Free PMC article.
-
Human breast tissue engineering in health and disease.EMBO Mol Med. 2024 Oct;16(10):2299-2321. doi: 10.1038/s44321-024-00112-3. Epub 2024 Aug 23. EMBO Mol Med. 2024. PMID: 39179741 Free PMC article. Review.
-
ECM-modulated cellular dynamics as a driving force for tissue morphogenesis.Curr Opin Genet Dev. 2013 Aug;23(4):408-14. doi: 10.1016/j.gde.2013.05.005. Epub 2013 Jul 9. Curr Opin Genet Dev. 2013. PMID: 23849799 Free PMC article. Review.
References
-
- Aman A, Piotrowski T (2010) Cell migration during morphogenesis. Dev Biol 341: 20–33 - PubMed
-
- Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132: 3151–3161 - PubMed
-
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

