Phosphorylation of CSF-1R Y721 mediates its association with PI3K to regulate macrophage motility and enhancement of tumor cell invasion
- PMID: 21610095
- PMCID: PMC3104034
- DOI: 10.1242/jcs.075309
Phosphorylation of CSF-1R Y721 mediates its association with PI3K to regulate macrophage motility and enhancement of tumor cell invasion
Abstract
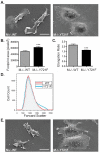
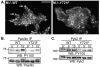
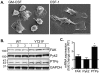
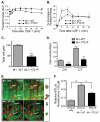
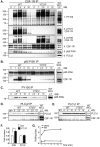
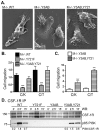
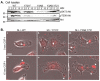
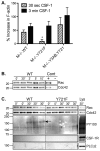
Colony stimulating factor-1 (CSF-1) regulates macrophage morphology and motility, as well as mononuclear phagocytic cell proliferation and differentiation. The CSF-1 receptor (CSF-1R) transduces these pleiotropic signals through autophosphorylation of eight intracellular tyrosine residues. We have used a novel bone-marrow-derived macrophage cell line system to examine specific signaling pathways activated by tyrosine-phosphorylated CSF-1R in macrophages. Screening of macrophages expressing a single species of CSF-1R with individual tyrosine-to-phenylalanine residue mutations revealed striking morphological alterations upon mutation of Y721. M⁻/⁻.Y721F cells were apolar and ruffled poorly in response to CSF-1. Y721-P-mediated CSF-1R signaling regulated adhesion and actin polymerization to control macrophage spreading and motility. Moreover, the reduced motility of M⁻/⁻.Y721F macrophages was associated with their reduced capacity to enhance carcinoma cell invasion. Y721 phosphorylation mediated the direct association of the p85 subunit of phosphoinositide 3-kinase (PI3K) with the CSF-1R, but not that of phospholipase C (PLC) γ2, and induced polarized PtdIns(3,4,5)P₃ production at the putative leading edge, implicating PI3K as a major regulator of CSF-1-induced macrophage motility. The Y721-P-motif-based motility signaling was at least partially independent of both Akt and increased Rac and Cdc42 activation but mediated the rapid and transient association of an unidentified ~170 kDa phosphorylated protein with either Rac-GTP or Cdc42-GTP. These studies identify CSF-1R-Y721-P-PI3K signaling as a major pathway in CSF-1-regulated macrophage motility and provide a starting point for the discovery of the immediate downstream signaling events.
Figures
Similar articles
-
Specific inhibition of PI3K p110δ inhibits CSF-1-induced macrophage spreading and invasive capacity.FEBS J. 2013 Nov;280(21):5228-36. doi: 10.1111/febs.12316. Epub 2013 Jun 5. FEBS J. 2013. PMID: 23648053 Free PMC article.
-
CSF-1 receptor structure/function in MacCsf1r-/- macrophages: regulation of proliferation, differentiation, and morphology.J Leukoc Biol. 2008 Sep;84(3):852-63. doi: 10.1189/jlb.0308171. Epub 2008 Jun 17. J Leukoc Biol. 2008. PMID: 18519746 Free PMC article.
-
Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1.J Bone Miner Res. 2005 Dec;20(12):2245-53. doi: 10.1359/JBMR.050816. Epub 2005 Aug 22. J Bone Miner Res. 2005. PMID: 16294277
-
Growth and differentiation signals regulated by the M-CSF receptor.Mol Reprod Dev. 1997 Jan;46(1):96-103. doi: 10.1002/(SICI)1098-2795(199701)46:1<96::AID-MRD15>3.0.CO;2-1. Mol Reprod Dev. 1997. PMID: 8981370 Review.
-
CSF-1 signaling in macrophages: pleiotrophy through phosphotyrosine-based signaling pathways.Crit Rev Clin Lab Sci. 2012 Mar-Apr;49(2):49-61. doi: 10.3109/10408363.2012.666845. Crit Rev Clin Lab Sci. 2012. PMID: 22468857 Review.
Cited by
-
The chemotactic defect in wiskott-Aldrich syndrome macrophages is due to the reduced persistence of directional protrusions.PLoS One. 2012;7(1):e30033. doi: 10.1371/journal.pone.0030033. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279563 Free PMC article.
-
A three-dimensional co-culture system to investigate macrophage-dependent tumor cell invasion.J Biol Methods. 2016 Jul 24;3(3):e49. doi: 10.14440/jbm.2016.132. eCollection 2016. J Biol Methods. 2016. PMID: 31453214 Free PMC article.
-
AZ304, a novel dual BRAF inhibitor, exerts anti-tumour effects in colorectal cancer independently of BRAF genetic status.Br J Cancer. 2018 May;118(11):1453-1463. doi: 10.1038/s41416-018-0086-x. Epub 2018 May 14. Br J Cancer. 2018. PMID: 29755114 Free PMC article.
-
Targeting Myeloid Cells in Combination Treatments for Glioma and Other Tumors.Front Immunol. 2019 Jul 23;10:1715. doi: 10.3389/fimmu.2019.01715. eCollection 2019. Front Immunol. 2019. PMID: 31396227 Free PMC article. Review.
-
Microglial cell loss after ischemic stroke favors brain neutrophil accumulation.Acta Neuropathol. 2019 Feb;137(2):321-341. doi: 10.1007/s00401-018-1954-4. Epub 2018 Dec 22. Acta Neuropathol. 2019. PMID: 30580383 Free PMC article.
References
-
- Allen W. E., Jones G. E., Pollard J. W., Ridley A. J. (1997). Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 110, 707-720 - PubMed
-
- Boocock C. A., Jones G. E., Stanley E. R., Pollard J. W. (1989). Colony-stimulating factor-1 induces rapid behavioural responses in the mouse macrophage cell line, BAC1. 2F5. J. Cell Sci. 93, 447-456 - PubMed
-
- Bunney T. D., Opaleye O., Roe S. M., Vatter P., Baxendale R. W., Walliser C., Everett K. L., Josephs M. B., Christow C., Rodrigues-Lima F., et al. (2009). Structural insights into formation of an active signaling complex between Rac and phospholipase C gamma 2. Mol. Cell 34, 223-233 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

