Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism
- PMID: 21606113
- PMCID: PMC3167122
- DOI: 10.1113/jphysiol.2011.207175
Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism
Abstract
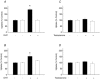
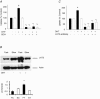
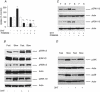
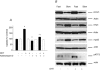
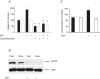
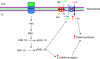
Dihydrotestosterone (DHT) has acute/non-genomic actions in adult mammalian skeletal muscles whose physiological functions are still poorly understood. Therefore, the primary aim of this study was to investigate the acute/non-genomic effects of DHT on amino acid uptake as well as the cellular signal transduction events underlying these actions in mouse fast- and slow-twitch skeletal muscle fibre bundles. 14C-Labelled amino acids were used to investigate the effects of DHT and testosterone (T) on amino acid uptake and pharmacological interventions were used to determine the cellular signal transduction events mediating these actions. While T had no effect on the uptake of isoleucine (Ile) and α-methylaminoisobutyric acid (MeAIB) in both fibre types, DHT increased their uptake in the fast-twitch fibre bundles. This effect was reversed by inhibitors of protein translation, the epidermal growth factor receptor (EGFR), system A, system L, mTOR and MEK. However, it was relatively insensitive to inhibitors of transcription, androgen receptors and PI3K/Akt. Additionally, DHT treatment increased the expression of LAT2 and the phosphorylation of the EGFR in the fast-twitch fibre bundles and that of ERK1/2, RSK1/2 and ATF2 in both fibre types. Also, it decreased the phosphorylation of eEF2 and increased the incorporation of Ile into proteins in both fibre types. Most of these effects were reversed by EGFR and MEK inhibitors. From these findings we suggest that another physiological function of the acute/non-genomic actions of DHT in isolated mammalian skeletal muscle fibres is to stimulate amino acid uptake. This effect is mediated through the EGFR and involves the activation of the MAPK pathway and an increase in LAT2 expression.
Figures
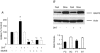
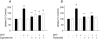
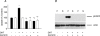
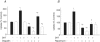
Similar articles
-
Dihydrotestosterone treatment rescues the decline in protein synthesis as a result of sarcopenia in isolated mouse skeletal muscle fibres.J Cachexia Sarcopenia Muscle. 2017 Feb;8(1):48-56. doi: 10.1002/jcsm.12122. Epub 2016 Apr 25. J Cachexia Sarcopenia Muscle. 2017. PMID: 27239418 Free PMC article.
-
Dihydrotestosterone activates the MAPK pathway and modulates maximum isometric force through the EGF receptor in isolated intact mouse skeletal muscle fibres.J Physiol. 2010 Feb 1;588(Pt 3):511-25. doi: 10.1113/jphysiol.2009.182162. Epub 2009 Dec 14. J Physiol. 2010. PMID: 20008468 Free PMC article.
-
Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy.Med Sci Sports Exerc. 2013 Sep;45(9):1712-20. doi: 10.1249/MSS.0b013e31828cf5f3. Med Sci Sports Exerc. 2013. PMID: 23470307
-
Evidence for a Non-Genomic Action of Testosterone in Skeletal Muscle Which may Improve Athletic Performance: Implications for the Female Athlete.J Sports Sci Med. 2012 Sep 1;11(3):363-70. J Sports Sci Med. 2012. PMID: 24149341 Free PMC article. Review.
-
Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions.Cell Mol Life Sci. 2012 May;69(10):1651-67. doi: 10.1007/s00018-011-0883-3. Epub 2011 Nov 19. Cell Mol Life Sci. 2012. PMID: 22101547 Free PMC article. Review.
Cited by
-
L-type amino acid transport and cancer: targeting the mTORC1 pathway to inhibit neoplasia.Am J Cancer Res. 2015 Mar 15;5(4):1281-94. eCollection 2015. Am J Cancer Res. 2015. PMID: 26101697 Free PMC article. Review.
-
Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated.Amino Acids. 2013 Sep;45(3):523-30. doi: 10.1007/s00726-012-1326-7. Epub 2012 May 30. Amino Acids. 2013. PMID: 22643846 Free PMC article.
-
The contradictory role of androgens in cutaneous and major burn wound healing.Burns Trauma. 2021 Apr 20;9:tkaa046. doi: 10.1093/burnst/tkaa046. eCollection 2021 Jan. Burns Trauma. 2021. PMID: 33928173 Free PMC article. Review.
-
Protein kinase C activation upregulates human L-type amino acid transporter 2 function.J Physiol Sci. 2021 Mar 31;71(1):11. doi: 10.1186/s12576-021-00795-0. J Physiol Sci. 2021. PMID: 33789576 Free PMC article.
-
SHBG is an important factor in stemness induction of cells by DHT in vitro and associated with poor clinical features of prostate carcinomas.PLoS One. 2013 Jul 30;8(7):e70558. doi: 10.1371/journal.pone.0070558. Print 2013. PLoS One. 2013. Retraction in: PLoS One. 2020 Aug 5;15(8):e0237487. doi: 10.1371/journal.pone.0237487. PMID: 23936228 Free PMC article. Retracted.
References
-
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. - PubMed
-
- Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab. 2009;297:E822–E829. - PubMed
-
- Barber EF, Handlogten ME, Vida TA, Kilberg MS. Neutral amino acid transport in hepatocytes isolated from streptozotocin-induced diabetic rats. J Biol Chem. 1982;257:14960–14967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

