Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection
- PMID: 21602490
- PMCID: PMC4201034
- DOI: 10.4049/jimmunol.1100823
Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection
Abstract
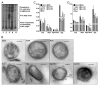
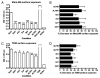
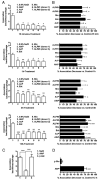
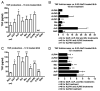
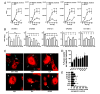
Pulmonary surfactant contains homeostatic and antimicrobial hydrolases. When Mycobacterium tuberculosis is initially deposited in the terminal bronchioles and alveoli, as well as following release from lysed macrophages, bacilli are in intimate contact with these lung surfactant hydrolases. We identified and measured several hydrolases in human alveolar lining fluid and lung tissue that, at their physiological concentrations, dramatically modified the M. tuberculosis cell envelope. Independent of their action time (15 min to 12 h), the effects of the hydrolases on the M. tuberculosis cell envelope resulted in a significant decrease (60-80%) in M. tuberculosis association with, and intracellular growth of the bacteria within, human macrophages. The cell envelope-modifying effects of the hydrolases also led to altered M. tuberculosis intracellular trafficking and induced a protective proinflammatory response to infection. These findings add a new concept to our understanding of M. tuberculosis-macrophage interactions (i.e., the impact of lung surfactant hydrolases on M. tuberculosis infection).
Figures
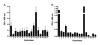
Similar articles
-
Deletion of PPARγ in lung macrophages provides an immunoprotective response against M. tuberculosis infection in mice.Tuberculosis (Edinb). 2018 Jul;111:170-177. doi: 10.1016/j.tube.2018.06.012. Epub 2018 Jun 21. Tuberculosis (Edinb). 2018. PMID: 30029904 Free PMC article.
-
A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth.Elife. 2020 Nov 24;9:e59961. doi: 10.7554/eLife.59961. Elife. 2020. PMID: 33228849 Free PMC article.
-
HDAC3 inhibitor RGFP966 controls bacterial growth and modulates macrophage signaling during Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2021 Mar;127:102062. doi: 10.1016/j.tube.2021.102062. Epub 2021 Feb 18. Tuberculosis (Edinb). 2021. PMID: 33639591 Free PMC article.
-
Macrophage immunoregulatory pathways in tuberculosis.Semin Immunol. 2014 Dec;26(6):471-85. doi: 10.1016/j.smim.2014.09.010. Epub 2014 Oct 30. Semin Immunol. 2014. PMID: 25453226 Free PMC article. Review.
-
Crosstalk between Mycobacterium tuberculosis and the host cell.Semin Immunol. 2014 Dec;26(6):486-96. doi: 10.1016/j.smim.2014.09.002. Epub 2014 Oct 7. Semin Immunol. 2014. PMID: 25303934 Free PMC article. Review.
Cited by
-
Molecular composition of the alveolar lining fluid in the aging lung.Age (Dordr). 2014 Jun;36(3):9633. doi: 10.1007/s11357-014-9633-4. Epub 2014 Mar 3. Age (Dordr). 2014. PMID: 24584696 Free PMC article.
-
Tuberculosis in the elderly: Why inflammation matters.Exp Gerontol. 2018 May;105:32-39. doi: 10.1016/j.exger.2017.12.021. Epub 2017 Dec 26. Exp Gerontol. 2018. PMID: 29287772 Free PMC article. Review.
-
HIV infection impairs the host response to Mycobacterium tuberculosis infection by altering surfactant protein D function in the human lung alveolar mucosa.Mucosal Immunol. 2024 Jun;17(3):461-475. doi: 10.1016/j.mucimm.2023.12.003. Epub 2024 Jan 4. Mucosal Immunol. 2024. PMID: 38184074 Free PMC article.
-
Identification of an Increased Alveolar Macrophage Subpopulation in Old Mice That Displays Unique Inflammatory Characteristics and Is Permissive to Mycobacterium tuberculosis Infection.J Immunol. 2019 Oct 15;203(8):2252-2264. doi: 10.4049/jimmunol.1900495. Epub 2019 Sep 11. J Immunol. 2019. PMID: 31511357 Free PMC article.
-
Antimicrobial Activity of Neutrophils Against Mycobacteria.Front Immunol. 2021 Dec 23;12:782495. doi: 10.3389/fimmu.2021.782495. eCollection 2021. Front Immunol. 2021. PMID: 35003097 Free PMC article. Review.
References
-
- World Health Organization. Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. WHO Press; Geneva, Switzerland: 2010.
-
- Schlesinger LS. The role of mononuclear phagocytes in tuberculosis. In: Lipscomb MF, Russell SW, editors. Lung macrophages and dendritic cells in health and disease. Marcel Dekker, Inc.; New York: 1997. pp. 437–480.
-
- King RJ. Pulmonary surfactant. J Appl Physiol. 1982;53:1–8. - PubMed
-
- vanGolde LM. Synthesis of surfactant lipids in the adult lung. Annu Rev Physiol. 1985;47:765–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

