Solution structure of the ESCRT-I complex by small-angle X-ray scattering, EPR, and FRET spectroscopy
- PMID: 21596998
- PMCID: PMC3111319
- DOI: 10.1073/pnas.1101763108
Solution structure of the ESCRT-I complex by small-angle X-ray scattering, EPR, and FRET spectroscopy
Abstract
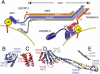
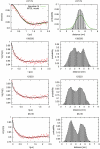
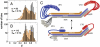
ESCRT-I is required for the sorting of integral membrane proteins to the lysosome, or vacuole in yeast, for cytokinesis in animal cells, and for the budding of HIV-1 from human macrophages and T lymphocytes. ESCRT-I is a heterotetramer of Vps23, Vps28, Vps37, and Mvb12. The crystal structures of the core complex and the ubiquitin E2 variant and Vps28 C-terminal domains have been determined, but internal flexibility has prevented crystallization of intact ESCRT-I. Here we have characterized the structure of ESCRT-I in solution by simultaneous structural refinement against small-angle X-ray scattering and double electron-electron resonance spectroscopy of spin-labeled complexes. An ensemble of at least six structures, comprising an equally populated mixture of closed and open conformations, was necessary to fit all of the data. This structural ensemble was cross-validated against single-molecule FRET spectroscopy, which suggested the presence of a continuum of open states. ESCRT-I in solution thus appears to consist of an approximately 50% population of one or a few related closed conformations, with the other 50% populating a continuum of open conformations. These conformations provide reference points for the structural pathway by which ESCRT-I induces membrane buds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

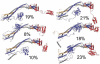
Similar articles
-
Solution structure of the ESCRT-I and -II supercomplex: implications for membrane budding and scission.Structure. 2012 May 9;20(5):874-86. doi: 10.1016/j.str.2012.03.008. Structure. 2012. PMID: 22579254 Free PMC article.
-
Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer.Cell. 2007 May 4;129(3):485-98. doi: 10.1016/j.cell.2007.03.016. Epub 2007 Apr 19. Cell. 2007. PMID: 17442384 Free PMC article.
-
Structural and functional organization of the ESCRT-I trafficking complex.Cell. 2006 Apr 7;125(1):113-26. doi: 10.1016/j.cell.2006.01.049. Cell. 2006. PMID: 16615894 Free PMC article.
-
Membrane budding and scission by the ESCRT machinery: it's all in the neck.Nat Rev Mol Cell Biol. 2010 Aug;11(8):556-66. doi: 10.1038/nrm2937. Epub 2010 Jun 30. Nat Rev Mol Cell Biol. 2010. PMID: 20588296 Free PMC article. Review.
-
Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes.Annu Rev Cell Dev Biol. 2018 Oct 6;34:85-109. doi: 10.1146/annurev-cellbio-100616-060600. Epub 2018 Aug 10. Annu Rev Cell Dev Biol. 2018. PMID: 30095293 Free PMC article. Review.
Cited by
-
FRET-based dynamic structural biology: Challenges, perspectives and an appeal for open-science practices.Elife. 2021 Mar 29;10:e60416. doi: 10.7554/eLife.60416. Elife. 2021. PMID: 33779550 Free PMC article. Review.
-
Investigating the Role of Large-Scale Domain Dynamics in Protein-Protein Interactions.Front Mol Biosci. 2016 Sep 13;3:54. doi: 10.3389/fmolb.2016.00054. eCollection 2016. Front Mol Biosci. 2016. PMID: 27679800 Free PMC article. Review.
-
Consistent View of Polypeptide Chain Expansion in Chemical Denaturants from Multiple Experimental Methods.J Am Chem Soc. 2016 Sep 14;138(36):11714-26. doi: 10.1021/jacs.6b05917. Epub 2016 Sep 1. J Am Chem Soc. 2016. PMID: 27583570 Free PMC article.
-
A helical assembly of human ESCRT-I scaffolds reverse-topology membrane scission.Nat Struct Mol Biol. 2020 Jun;27(6):570-580. doi: 10.1038/s41594-020-0426-4. Epub 2020 May 18. Nat Struct Mol Biol. 2020. PMID: 32424346 Free PMC article.
-
Membrane fluctuations and acidosis regulate cooperative binding of 'marker of self' protein CD47 with the macrophage checkpoint receptor SIRPα.J Cell Sci. 2018 Jul 16;132(4):jcs216770. doi: 10.1242/jcs.216770. J Cell Sci. 2018. PMID: 29777034 Free PMC article.
References
-
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. - PubMed
-
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

