Hippocampal sclerosis in advanced age: clinical and pathological features
- PMID: 21596774
- PMCID: PMC3097889
- DOI: 10.1093/brain/awr053
Hippocampal sclerosis in advanced age: clinical and pathological features
Abstract
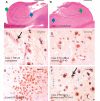
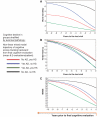
Hippocampal sclerosis is a relatively common neuropathological finding (∼10% of individuals over the age of 85 years) characterized by cell loss and gliosis in the hippocampus that is not explained by Alzheimer's disease. Hippocampal sclerosis pathology can be associated with different underlying causes, and we refer to hippocampal sclerosis in the aged brain as hippocampal sclerosis associated with ageing. Much remains unknown about hippocampal sclerosis associated with ageing. We combined three different large autopsy cohorts: University of Kentucky Alzheimer's Disease Centre, the Nun Study and the Georgia Centenarian Study to obtain a pool of 1110 patients, all of whom were evaluated neuropathologically at the University of Kentucky. We focused on the subset of cases with neuropathology-confirmed hippocampal sclerosis (n=106). For individuals aged≥95 years at death (n=179 in our sample), each year of life beyond the age of 95 years correlated with increased prevalence of hippocampal sclerosis pathology and decreased prevalence of 'definite' Alzheimer's disease pathology. Aberrant TAR DNA protein 43 immunohistochemistry was seen in 89.9% of hippocampal sclerosis positive patients compared with 9.7% of hippocampal sclerosis negative patients. TAR DNA protein 43 immunohistochemistry can be used to demonstrate that the disease is usually bilateral even when hippocampal sclerosis pathology is not obvious by haematoxylin and eosin stains. TAR DNA protein 43 immunohistochemistry was negative on brain sections from younger individuals (n=10) after hippocampectomy due to seizures, who had pathologically confirmed hippocampal sclerosis. There was no association between cases with hippocampal sclerosis associated with ageing and apolipoprotein E genotype. Age of death and clinical features of hippocampal sclerosis associated with ageing (with or without aberrant TAR DNA protein 43) were distinct from previously published cases of frontotemporal lobar degeneration TAR DNA protein 43. To help sharpen our ability to discriminate patients with hippocampal sclerosis associated with ageing clinically, the longitudinal cognitive profile of 43 patients with hippocampal sclerosis associated with ageing was compared with the profiles of 75 controls matched for age, gender, education level and apolipoprotein E genotype. These individuals were followed from intake assessment, with 8.2 (average) longitudinal cognitive assessments. A neuropsychological profile with relatively high-verbal fluency but low word list recall distinguished the hippocampal sclerosis associated with ageing group at intake (P<0.015) and also 5.5-6.5 years before death (P<0.005). This may provide a first step in clinical differentiation of hippocampal sclerosis associated with ageing versus pure Alzheimer's disease in their earliest stages. In summary, in the largest series of autopsy-verified patients with hippocampal sclerosis to date, we characterized the clinical and pathological features associated with hippocampal sclerosis associated with ageing.
Figures

Comment in
-
Diagnosis of pure subcortical vascular dementia, hippocampal sclerosis in advanced age and imaging of training-related brain plasticity by fMRI.J Neurol. 2011 Aug;258(8):1573-6. doi: 10.1007/s00415-011-6184-9. J Neurol. 2011. PMID: 21796414 No abstract available.
Similar articles
-
Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing.Brain. 2014 Jan;137(Pt 1):255-67. doi: 10.1093/brain/awt318. Epub 2013 Nov 21. Brain. 2014. PMID: 24271328 Free PMC article.
-
Hippocampal sclerosis of aging is a key Alzheimer's disease mimic: clinical-pathologic correlations and comparisons with both alzheimer's disease and non-tauopathic frontotemporal lobar degeneration.J Alzheimers Dis. 2014;39(3):691-702. doi: 10.3233/JAD-131880. J Alzheimers Dis. 2014. PMID: 24270205 Free PMC article.
-
TDP-43 pathological changes in early onset familial and sporadic Alzheimer's disease, late onset Alzheimer's disease and Down's syndrome: association with age, hippocampal sclerosis and clinical phenotype.Acta Neuropathol. 2011 Dec;122(6):703-13. doi: 10.1007/s00401-011-0879-y. Epub 2011 Oct 4. Acta Neuropathol. 2011. PMID: 21968532
-
Hippocampal Sclerosis of Aging Can Be Segmental: Two Cases and Review of the Literature.J Neuropathol Exp Neurol. 2015 Jul;74(7):642-52. doi: 10.1097/NEN.0000000000000204. J Neuropathol Exp Neurol. 2015. PMID: 26083567 Free PMC article. Review.
-
"New Old Pathologies": AD, PART, and Cerebral Age-Related TDP-43 With Sclerosis (CARTS).J Neuropathol Exp Neurol. 2016 Jun;75(6):482-98. doi: 10.1093/jnen/nlw033. Epub 2016 May 21. J Neuropathol Exp Neurol. 2016. PMID: 27209644 Free PMC article. Review.
Cited by
-
Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging.Acta Neuropathol Commun. 2015 May 23;3:32. doi: 10.1186/s40478-015-0209-z. Acta Neuropathol Commun. 2015. PMID: 26001591 Free PMC article.
-
TAR DNA-binding protein 43 and pathological subtype of Alzheimer's disease impact clinical features.Ann Neurol. 2015 Nov;78(5):697-709. doi: 10.1002/ana.24493. Epub 2015 Sep 16. Ann Neurol. 2015. PMID: 26224156 Free PMC article.
-
The Neuropathological Diagnosis of Alzheimer's Disease-The Challenges of Pathological Mimics and Concomitant Pathology.Brain Sci. 2020 Jul 24;10(8):479. doi: 10.3390/brainsci10080479. Brain Sci. 2020. PMID: 32722332 Free PMC article. Review.
-
Detection of partial loss of hippocampal striation at 1.5 Tesla magnetic resonance imaging.Insights Imaging. 2019 Oct 26;10(1):103. doi: 10.1186/s13244-019-0783-x. Insights Imaging. 2019. PMID: 31655929 Free PMC article.
-
Clinico-Neuropathological Findings in the Oldest Old from the Georgia Centenarian Study.J Alzheimers Dis. 2019;70(1):35-49. doi: 10.3233/JAD-181110. J Alzheimers Dis. 2019. PMID: 31177211 Free PMC article.
References
-
- Ala TA, Beh GO, Frey WH., 2nd Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;54:843–8. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

