Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity
- PMID: 21593203
- PMCID: PMC3121443
- DOI: 10.2337/db11-0030
Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity
Abstract
Objective: Bile acids (BA) participate in the maintenance of metabolic homeostasis acting through different signaling pathways. The nuclear BA receptor farnesoid X receptor (FXR) regulates pathways in BA, lipid, glucose, and energy metabolism, which become dysregulated in obesity. However, the role of FXR in obesity and associated complications, such as dyslipidemia and insulin resistance, has not been directly assessed.
Research design and methods: Here, we evaluate the consequences of FXR deficiency on body weight development, lipid metabolism, and insulin resistance in murine models of genetic and diet-induced obesity.
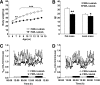
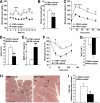
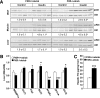
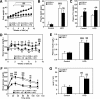
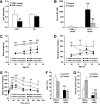
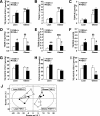
Results: FXR deficiency attenuated body weight gain and reduced adipose tissue mass in both models. Surprisingly, glucose homeostasis improved as a result of an enhanced glucose clearance and adipose tissue insulin sensitivity. In contrast, hepatic insulin sensitivity did not change, and liver steatosis aggravated as a result of the repression of β-oxidation genes. In agreement, liver-specific FXR deficiency did not protect from diet-induced obesity and insulin resistance, indicating a role for nonhepatic FXR in the control of glucose homeostasis in obesity. Decreasing elevated plasma BA concentrations in obese FXR-deficient mice by administration of the BA sequestrant colesevelam improved glucose homeostasis in a FXR-dependent manner, indicating that the observed improvements by FXR deficiency are not a result of indirect effects of altered BA metabolism.
Conclusions: Overall, FXR deficiency in obesity beneficially affects body weight development and glucose homeostasis.
© 2011 by the American Diabetes Association.
Figures


Similar articles
-
The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice.J Biol Chem. 2006 Apr 21;281(16):11039-49. doi: 10.1074/jbc.M510258200. Epub 2006 Jan 30. J Biol Chem. 2006. PMID: 16446356
-
Bile acids as regulators of hepatic lipid and glucose metabolism.Dig Dis. 2010;28(1):220-4. doi: 10.1159/000282091. Epub 2010 May 7. Dig Dis. 2010. PMID: 20460915 Review.
-
Microbiota-induced obesity requires farnesoid X receptor.Gut. 2017 Mar;66(3):429-437. doi: 10.1136/gutjnl-2015-310283. Epub 2016 Jan 6. Gut. 2017. PMID: 26740296 Free PMC article.
-
Adipocyte-specific FXR-deficiency protects adipose tissue from oxidative stress and insulin resistance and improves glucose homeostasis.Mol Metab. 2023 Mar;69:101686. doi: 10.1016/j.molmet.2023.101686. Epub 2023 Feb 4. Mol Metab. 2023. PMID: 36746333 Free PMC article.
-
Role of bile acids and bile acid receptors in metabolic regulation.Physiol Rev. 2009 Jan;89(1):147-91. doi: 10.1152/physrev.00010.2008. Physiol Rev. 2009. PMID: 19126757 Review.
Cited by
-
The significance of the nuclear farnesoid X receptor (FXR) in β cell function.Islets. 2012 Sep-Oct;4(5):333-8. doi: 10.4161/isl.22383. Epub 2012 Sep 1. Islets. 2012. PMID: 23073079 Free PMC article. Review.
-
The Farnesoid X Receptor as a Master Regulator of Hepatotoxicity.Int J Mol Sci. 2022 Nov 12;23(22):13967. doi: 10.3390/ijms232213967. Int J Mol Sci. 2022. PMID: 36430444 Free PMC article. Review.
-
Correlated Responses to Selection for Intramuscular Fat on the Gut Microbiome in Rabbits.Animals (Basel). 2024 Jul 16;14(14):2078. doi: 10.3390/ani14142078. Animals (Basel). 2024. PMID: 39061540 Free PMC article.
-
Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk.J Clin Invest. 2014 Mar;124(3):1037-51. doi: 10.1172/JCI68815. Epub 2014 Feb 17. J Clin Invest. 2014. PMID: 24531544 Free PMC article.
-
Glucagon Receptor Signaling Regulates Energy Metabolism via Hepatic Farnesoid X Receptor and Fibroblast Growth Factor 21.Diabetes. 2018 Sep;67(9):1773-1782. doi: 10.2337/db17-1502. Epub 2018 Jun 20. Diabetes. 2018. PMID: 29925501 Free PMC article.
References
-
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009;89:147–191 - PubMed
-
- Prawitt J, Caron S, Staels B. How to modulate FXR activity to treat the metabolic syndrome. Drug Discov Today Dis Mech 2009;6:e55–e64
-
- Kast HR, Nguyen CM, Sinal CJ, et al. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol 2001;15:1720–1728 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

