Induction of tolerance to factor VIII by transient co-administration with rapamycin
- PMID: 21585650
- PMCID: PMC3154987
- DOI: 10.1111/j.1538-7836.2011.04351.x
Induction of tolerance to factor VIII by transient co-administration with rapamycin
Abstract
Background: Formation of inhibitory antibodies is a frequent and serious complication of factor (F) VIII replacement therapy for the X-linked bleeding disorder hemophilia A. Similarly, hemophilia A mice develop high-titer inhibitors to recombinant human FVIII after a few intravenous injections.
Objective: Using the murine model, the study sought to develop a short regimen capable of inducing tolerance to FVIII.
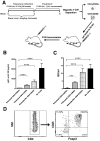
Methods: A 1-month immunomodulatory protocol, consisting of FVIII administration combined with oral delivery of rapamycin, was developed.
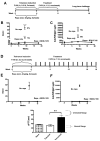
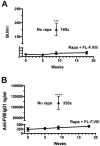
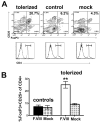
Results: The protocol effectively prevented formation of inhibitors to FVIII upon subsequent intravenous treatment (weekly for 3.5 months). Control mice formed high-titer inhibitors and had CD4(+) T effector cell responses characterized by expression of IL-2, IL-4 and IL-6. Tolerized mice instead had a CD4(+)CD25(+)FoxP3(+) T cell response to FVIII that suppressed antibody formation upon adoptive transfer, indicating a shift from Th2 to Treg if FVIII antigen was introduced to T cells during inhibition with rapamycin. CD4(+) T cells from tolerized mice also expressed TGF-β1 and CTLA4, but not IL-10. The presence of FVIII antigen during the time of rapamycin administration was required for specific tolerance induction.
Conclusions: The study shows that a prophylactic immune tolerance protocol for FVIII can be developed using rapamycin, a drug that is already widely in clinical application. Immune suppression with rapamycin was mild and highly transient, as the mice regained immune competence within a few weeks.
© 2011 International Society on Thrombosis and Haemostasis.
Figures



Comment in
-
Tilt balance towards regulation: evolving new strategy for treatment of hemophilia inhibitors.J Thromb Haemost. 2011 Aug;9(8):1521-3. doi: 10.1111/j.1538-7836.2011.04391.x. J Thromb Haemost. 2011. PMID: 21649848 Free PMC article. No abstract available.
Similar articles
-
Long-term tolerance to factor VIII is achieved by administration of interleukin-2/interleukin-2 monoclonal antibody complexes and low dosages of factor VIII.J Thromb Haemost. 2014 Jun;12(6):921-31. doi: 10.1111/jth.12576. J Thromb Haemost. 2014. PMID: 24684505 Free PMC article.
-
Combination therapy for inhibitor reversal in haemophilia A using monoclonal anti-CD20 and rapamycin.Thromb Haemost. 2017 Jan 5;117(1):33-43. doi: 10.1160/TH16-05-0404. Epub 2016 Sep 29. Thromb Haemost. 2017. PMID: 27683758 Free PMC article.
-
B Cell Depletion Eliminates FVIII Memory B Cells and Enhances AAV8-coF8 Immune Tolerance Induction When Combined With Rapamycin.Front Immunol. 2020 Jun 24;11:1293. doi: 10.3389/fimmu.2020.01293. eCollection 2020. Front Immunol. 2020. PMID: 32670285 Free PMC article.
-
Endocytic receptor for pro-coagulant factor VIII: relevance to inhibitor formation.Thromb Haemost. 2010 Dec;104(6):1093-8. doi: 10.1160/TH10-05-0294. Epub 2010 Sep 30. Thromb Haemost. 2010. PMID: 20886186 Review.
-
Dangerous liaisons: how the immune system deals with factor VIII.J Thromb Haemost. 2013 Jan;11(1):47-55. doi: 10.1111/jth.12065. J Thromb Haemost. 2013. PMID: 23140211 Review.
Cited by
-
Cytokine-conditioned dendritic cells induce humoral tolerance to protein therapy in mice.Hum Gene Ther. 2012 Jul;23(7):769-80. doi: 10.1089/hum.2011.225. Epub 2012 May 11. Hum Gene Ther. 2012. PMID: 22468961 Free PMC article.
-
Modification of an exposed loop in the C1 domain reduces immune responses to factor VIII in hemophilia A mice.Blood. 2012 May 31;119(22):5294-300. doi: 10.1182/blood-2011-11-391680. Epub 2012 Apr 12. Blood. 2012. PMID: 22498747 Free PMC article.
-
Pre-Clinical Assessment of Immune Responses to Adeno-Associated Virus (AAV) Vectors.Front Immunol. 2014 Feb 7;5:28. doi: 10.3389/fimmu.2014.00028. eCollection 2014. Front Immunol. 2014. PMID: 24570676 Free PMC article. Review.
-
A Treg-Selective IL-2 Mutein Prevents the Formation of Factor VIII Inhibitors in Hemophilia Mice Treated With Factor VIII Gene Therapy.Front Immunol. 2020 Apr 28;11:638. doi: 10.3389/fimmu.2020.00638. eCollection 2020. Front Immunol. 2020. PMID: 32411127 Free PMC article.
-
Copackaged AAV9 Vectors Promote Simultaneous Immune Tolerance and Phenotypic Correction of Pompe Disease.Hum Gene Ther. 2016 Jan;27(1):43-59. doi: 10.1089/hum.2015.103. Hum Gene Ther. 2016. PMID: 26603344 Free PMC article.
References
-
- DiMichele DM. Immune tolerance: critical issues of factor dose, purity and treatment complications. Haemophilia. 2006;12(Suppl 6):81–5. discussion 5-6. - PubMed
-
- Ghosh K, Shetty S. Immune Response to FVIII in Hemophilia A: An Overview of Risk Factors. Clin Rev Allergy Immunol. 2009;37:58–66. - PubMed
-
- Coppola A, Santoro C, Tagliaferri A, Franchini M, G DIM. Understanding inhibitor development in haemophilia A: towards clinical prediction and prevention strategies. Haemophilia. 2010;16(Suppl 1):13–9. - PubMed
-
- Peerlinck K, Jacquemin M. Mild haemophilia: a disease with many faces and many unexpected pitfalls. Haemophilia. 2010;16(Suppl 5):100–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

