A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus
- PMID: 21575912
- PMCID: PMC3100664
- DOI: 10.1016/j.chom.2011.04.007
A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus
Abstract
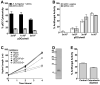
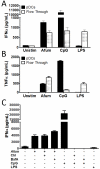
While plasmacytoid dendritic cells (pDCs), a natural type I interferon (IFN)-producing cell type, are regarded as critical for innate immunity to viruses, their role in defense against fungal infections remains unknown. We examined the interactions of pDCs with hyphae of the invasive human fungal pathogen Aspergillus fumigatus. Human pDCs spread over hyphae and inhibited their growth. Antifungal activity was retained in pDC lysates, did not require direct fungal contact, and was partially reversed by zinc. Incubation with hyphae resulted in pDC cytotoxicity, partly due to fungal gliotoxin secretion. Following hyphal stimulation, pDCs released proinflammatory cytokines via a TLR9-independent mechanism. Pulmonary challenge of mice with A. fumigatus resulted in a substantial influx of pDCs into lungs, and pDC-depleted mice were hypersusceptible to invasive aspergillosis. These data demonstrate the antifungal activity of pDCs against A. fumigatus and establish their nonredundant role in host defenses against invasive aspergillosis in vivo.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

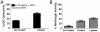
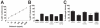


Similar articles
-
Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps.PLoS Pathog. 2015 Feb 6;11(2):e1004643. doi: 10.1371/journal.ppat.1004643. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25659141 Free PMC article.
-
During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk.Cell Host Microbe. 2020 Jul 8;28(1):104-116.e4. doi: 10.1016/j.chom.2020.05.002. Epub 2020 Jun 1. Cell Host Microbe. 2020. PMID: 32485165 Free PMC article.
-
Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease.Infect Immun. 2008 Aug;76(8):3429-38. doi: 10.1128/IAI.01510-07. Epub 2008 May 19. Infect Immun. 2008. PMID: 18490455 Free PMC article.
-
Control, immunoregulation, and expression of innate pulmonary host defenses against Aspergillus fumigatus.Med Mycol. 2005 May;43 Suppl 1:S165-72. doi: 10.1080/13693780500064672. Med Mycol. 2005. PMID: 16114132 Review.
-
Pulmonary defense mechanisms against opportunistic fungal pathogens.Immunol Ser. 1989;47:243-71. Immunol Ser. 1989. PMID: 2490078 Review.
Cited by
-
Toll-like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae.Infect Immun. 2011 Dec;79(12):4858-67. doi: 10.1128/IAI.05626-11. Epub 2011 Sep 26. Infect Immun. 2011. PMID: 21947771 Free PMC article.
-
Preparations for Invasion: Modulation of Host Lung Immunity During Pulmonary Aspergillosis by Gliotoxin and Other Fungal Secondary Metabolites.Front Immunol. 2018 Nov 6;9:2549. doi: 10.3389/fimmu.2018.02549. eCollection 2018. Front Immunol. 2018. PMID: 30459771 Free PMC article. Review.
-
Pathogenesis of Respiratory Viral and Fungal Coinfections.Clin Microbiol Rev. 2022 Jan 19;35(1):e0009421. doi: 10.1128/CMR.00094-21. Epub 2021 Nov 17. Clin Microbiol Rev. 2022. PMID: 34788127 Free PMC article. Review.
-
Immunoregulation in Fungal Diseases.Microorganisms. 2016 Dec 10;4(4):47. doi: 10.3390/microorganisms4040047. Microorganisms. 2016. PMID: 27973396 Free PMC article. Review.
-
The role of DC subgroups in the pathogenesis of asthma.Front Immunol. 2024 Oct 28;15:1481989. doi: 10.3389/fimmu.2024.1481989. eCollection 2024. Front Immunol. 2024. PMID: 39530090 Free PMC article. Review.
References
-
- Ang DK, Oates CV, Schuelein R, Kelly M, Sansom FM, Bourges D, Boon L, Hertzog PJ, Hartland EL, van Driel IR. Cutting edge: Pulmonary Legionella pneumophila is controlled by plasmacytoid dendritic cells but not type I IFN. J Immunol. 2010;184:5429–5433. - PubMed
-
- Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. - PubMed
-
- Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. - PubMed
-
- Biondo C, Midiri A, Gambuzza M, Gerace E, Falduto M, Galbo R, Bellantoni A, Beninati C, Teti G, Leanderson T, Mancuso G. IFN-alpha/beta signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J Immunol. 2008;181:566–573. - PubMed
-
- Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone Marrow Stromal Cell Antigen 2 Is a Specific Marker of Type I IFN-Producing Cells in the Naive Mouse, but a Promiscuous Cell Surface Antigen following IFN Stimulation. The Journal of Immunology. 2006;177:3260–3265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

