miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner
- PMID: 21575235
- PMCID: PMC3120797
- DOI: 10.1186/1476-4598-10-55
miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner
Abstract
Background: microRNAs (miRNAs) are small non-coding RNAs that are frequently involved in carcinogenesis. Although many miRNAs form part of integrated networks, little information is available how they interact with each other to control cellular processes. miR-34a and miR-15a/16 are functionally related; they share common targets and control similar processes including G1-S cell cycle progression and apoptosis. The aim of this study was to investigate the combined action of miR-34a and miR-15a/16 in non-small cell lung cancer (NSCLC) cells.
Methods: NSCLC cells were transfected with miR-34a and miR-15a/16 mimics and analysed for cell cycle arrest and apoptosis by flow cytometry. Expression of retinoblastoma and cyclin E1 was manipulated to investigate the role of these proteins in miRNA-induced cell cycle arrest. Expression of miRNA targets was assessed by real-time PCR. To investigate if both miRNAs are co-regulated in NSCLC cells, tumour tissue and matched normal lung tissue from 23 patients were collected by laser capture microdissection and compared for the expression of these miRNAs by real-time PCR.
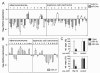
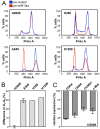
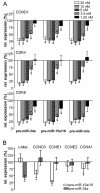
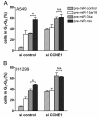
Results: In the present study, we demonstrate that miR-34a and miR-15a/16 act synergistically to induce cell cycle arrest in a Rb-dependent manner. In contrast, no synergistic effect of these miRNAs was observed for apoptosis. The synergistic action on cell cycle arrest was not due to a more efficient down-regulation of targets common to both miRNAs. However, the synergistic effect was abrogated in cells in which cyclin E1, a target unique to miR-15a/16, was silenced by RNA interference. Thus, the synergistic effect was due to the fact that in concerted action both miRNAs are able to down-regulate more targets involved in cell cycle control than each miRNA alone. Both miRNAs were significantly co-regulated in adenocarcinomas of the lung suggesting a functional link between these miRNAs.
Conclusions: In concerted action miRNAs are able to potentiate their impact on G1-S progression. Thus the combination of miRNAs of the same network rather than individual miRNAs should be considered for assessing a biological response. Since miR-34a and miR-15a/16 are frequently down-regulated in the same tumour tissue, administrating a combination of both miRNAs may also potentiate their therapeutic impact.
Figures



Similar articles
-
MicroRNAs in non-small cell lung cancer: Gene regulation, impact on cancer cellular processes, and therapeutic potential.Pharmacol Res Perspect. 2019 Dec;7(6):e00528. doi: 10.1002/prp2.528. Pharmacol Res Perspect. 2019. PMID: 31859460 Free PMC article. Review.
-
miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer.Cancer Res. 2009 Jul 1;69(13):5553-9. doi: 10.1158/0008-5472.CAN-08-4277. Epub 2009 Jun 23. Cancer Res. 2009. PMID: 19549910
-
The RNA-binding protein SART3 promotes miR-34a biogenesis and G1 cell cycle arrest in lung cancer cells.J Biol Chem. 2019 Nov 15;294(46):17188-17196. doi: 10.1074/jbc.AC119.010419. Epub 2019 Oct 16. J Biol Chem. 2019. PMID: 31619517 Free PMC article.
-
miR-150, p53 protein and relevant miRNAs consist of a regulatory network in NSCLC tumorigenesis.Oncol Rep. 2013 Jul;30(1):492-8. doi: 10.3892/or.2013.2453. Epub 2013 May 13. Oncol Rep. 2013. PMID: 23670238
-
Prospects of microRNAs as therapeutic biomarkers in non-small cell lung cancer.Med Oncol. 2023 Nov 3;40(12):345. doi: 10.1007/s12032-023-02212-5. Med Oncol. 2023. PMID: 37922117 Review.
Cited by
-
MicroRNA-34a affects the occurrence of laryngeal squamous cell carcinoma by targeting the antiapoptotic gene survivin.Med Oncol. 2012 Dec;29(4):2473-80. doi: 10.1007/s12032-011-0156-x. Epub 2012 Jan 14. Med Oncol. 2012. PMID: 22246523
-
MicroRNAs in non-small cell lung cancer: Gene regulation, impact on cancer cellular processes, and therapeutic potential.Pharmacol Res Perspect. 2019 Dec;7(6):e00528. doi: 10.1002/prp2.528. Pharmacol Res Perspect. 2019. PMID: 31859460 Free PMC article. Review.
-
Microribonucleic Acid-15a-5p Alters Adriamycin Resistance in Breast Cancer Cells by Targeting Cell Division Cycle-Associated Protein 4.Cancer Manag Res. 2021 Nov 10;13:8425-8434. doi: 10.2147/CMAR.S333830. eCollection 2021. Cancer Manag Res. 2021. PMID: 34785950 Free PMC article.
-
miR-34a inhibits tumorigenesis of NSCLC via targeting SIRT6.Int J Clin Exp Pathol. 2018 Mar 1;11(3):1135-1145. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31938208 Free PMC article.
-
The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells.PLoS One. 2014 Mar 4;9(3):e90022. doi: 10.1371/journal.pone.0090022. eCollection 2014. PLoS One. 2014. PMID: 24595209 Free PMC article.
References
-
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RN, Pickle LW. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. - DOI - PubMed
-
- Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. - DOI - PubMed

