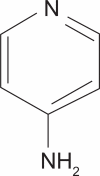
Impact of extended-release dalfampridine on walking ability in patients with multiple sclerosis
- PMID: 21573085
- PMCID: PMC3090287
- DOI: 10.2147/NDT.S10469
Impact of extended-release dalfampridine on walking ability in patients with multiple sclerosis
Abstract
Dalfampridine extended release (ER) 10 mg is an oral tablet form of the potassium (K(+)) channel-blocking compounded dalfampridine, also known as fampridine, and chemically 4-aminopyridine or 4-AP, which received regulatory approval in the United States for the treatment of walking in patients with multiple sclerosis (MS) in January 2010. Two pivotal Phase 3 clinical trials demonstrated significant improvements in walking in patients with the four primary forms of MS following administration of dalfampridine ER tablets 10 mg twice daily. The drug is thought to act by restoring conduction in focally demyelinated axons and by enhancing neurotransmission, thereby leading to improved neurological function. This review describes how dalfampridine represents a new pharmacotherapeutic approach to the clinical management of mobility impairment. It describes the mechanism of action and chemistry of dalfampridine ER, its pharmacokinetics, tolerability, and side effects, and the outcomes of multicenter trials showing its efficacy in improving walking speed. Clinician and patient global assessments, as well as patient self-assessment of the impact of MS on their gait disability, confirm clinically relevant benefit from the therapy. Patients tolerate the drug well and their improvement in terms of household and community ambulation, inferred from analysis of pooled data from several studies, is likely to translate into benefits in the performance of instrumental activities of daily living and a reduction in the neuropsychiatric burden of disease.
Keywords: 4-aminopyridine; Ampyra®; dalfampridine ER; demyelination; fampridine-SR; multiple sclerosis.
Figures
Similar articles
-
Development of dalfampridine, a novel pharmacologic approach for treating walking impairment in multiple sclerosis.Ann N Y Acad Sci. 2014 Nov;1329:33-44. doi: 10.1111/nyas.12512. Epub 2014 Aug 25. Ann N Y Acad Sci. 2014. PMID: 25154911 Review.
-
Dalfampridine: a brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis.Curr Med Res Opin. 2011 Jul;27(7):1415-23. doi: 10.1185/03007995.2011.583229. Epub 2011 May 20. Curr Med Res Opin. 2011. PMID: 21595605 Review.
-
Treatment of walking impairment in multiple sclerosis with dalfampridine.Ther Adv Neurol Disord. 2011 Mar;4(2):99-109. doi: 10.1177/1756285611403960. Ther Adv Neurol Disord. 2011. PMID: 21694807 Free PMC article.
-
Walking impairment in patients with multiple sclerosis - a new therapeutic approach and clinical potential of dalfampridine extended release tablets.Degener Neurol Neuromuscul Dis. 2012 Jun 22;2:53-64. doi: 10.2147/DNND.S19839. eCollection 2012. Degener Neurol Neuromuscul Dis. 2012. PMID: 30890878 Free PMC article. Review.
-
Dalfampridine extended release: in multiple sclerosis.CNS Drugs. 2010 Oct;24(10):883-91. doi: 10.2165/11205910-000000000-00000. CNS Drugs. 2010. PMID: 20839898 Review.
Cited by
-
Critical Appraisal of Evidence for Improving Gait Speed in People with Multiple Sclerosis: Dalfampridine Versus Gait Training.Int J MS Care. 2016 May-Jun;18(3):105-15. doi: 10.7224/1537-2073.2014-114. Int J MS Care. 2016. PMID: 27252597 Free PMC article.
-
The role of exercise in modifying outcomes for people with multiple sclerosis: a randomized trial.BMC Neurol. 2013 Jun 28;13:69. doi: 10.1186/1471-2377-13-69. BMC Neurol. 2013. PMID: 23809312 Free PMC article. Clinical Trial.
-
4-Aminopyridine for symptomatic treatment of multiple sclerosis: a systematic review.Ther Adv Neurol Disord. 2014 Mar;7(2):97-113. doi: 10.1177/1756285613512712. Ther Adv Neurol Disord. 2014. PMID: 24587826 Free PMC article. Review.
-
Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review).Mol Med Rep. 2021 Jun;23(6):417. doi: 10.3892/mmr.2021.12056. Epub 2021 Apr 13. Mol Med Rep. 2021. PMID: 33846780 Free PMC article. Review.
-
Improving Detection of Change in Motor Functioning in Multiple Sclerosis Using Video-Assisted Composite Measures.Int J MS Care. 2022 Sep-Oct;24(5):230-234. doi: 10.7224/1537-2073.2021-044. Epub 2022 Jun 20. Int J MS Care. 2022. PMID: 36090237 Free PMC article.
References
-
- Baum HM, Rothschild BB. Multiple sclerosis and mobility restriction. Arch Phys Med Rehabil. 1983;64:591–596. - PubMed
-
- Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010;26:109–119. - PubMed
-
- Myhr KM, Riise T, Vedeler C, et al. Disability and prognosis in multiple sclerosis: demographic and clinical variables important for the ability to walk and awarding of disability pension. Mult Scler. 2001;7:59–65. - PubMed
-
- Whetten-Goldstein K, Sloan FA, Goldstein LB, et al. A comprehensive assessment of the cost of multiple sclerosis in the United states. Mult Scler. 1998;4:419–425. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials