Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG)
- PMID: 21571150
- PMCID: PMC3133619
- DOI: 10.1016/S0140-6736(11)60355-3
Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG)
Abstract
Background: Sickle-cell anaemia is associated with substantial morbidity from acute complications and organ dysfunction beginning in the first year of life. Hydroxycarbamide substantially reduces episodes of pain and acute chest syndrome, admissions to hospital, and transfusions in adults with sickle-cell anaemia. We assessed the effect of hydroxycarbamide therapy on organ dysfunction and clinical complications, and examined laboratory findings and toxic effects.
Methods: This randomised trial was undertaken in 13 centres in the USA between October, 2003, and September, 2009. Eligible participants had haemoglobin SS (HbSS) or haemoglobin Sβ(0)thalassaemia, were aged 9-18 months at randomisation, and were not selected for clinical severity. Participants received liquid hydroxycarbamide, 20 mg/kg per day, or placebo for 2 years. Randomisation assignments were generated by the medical coordinating centre by a pre-decided schedule. Identical appearing and tasting formulations were used for hydroxycarbamide and placebo. Patients, caregivers, and coordinating centre staff were masked to treatment allocation. Primary study endpoints were splenic function (qualitative uptake on (99)Tc spleen scan) and renal function (glomerular filtration rate by (99m)Tc-DTPA clearance). Additional assessments included blood counts, fetal haemoglobin concentration, chemistry profiles, spleen function biomarkers, urine osmolality, neurodevelopment, transcranial Doppler ultrasonography, growth, and mutagenicity. Study visits occurred every 2-4 weeks. Analysis was by intention to treat. The trial is registered with ClinicalTrials.gov, number NCT00006400.
Findings: 96 patients received hydroxycarbamide and 97 placebo, of whom 83 patients in the hydroxycarbamide group and 84 in the placebo group completed the study. Significant differences were not seen between groups for the primary endpoints (19 of 70 patients with decreased spleen function at exit in the hydroxycarbamide group vs 28 of 74 patients in the placebo group, p=0·21; and a difference in the mean increase in DTPA glomerular filtration rate in the hydroxycarbamide group versus the placebo group of 2 mL/min per 1·73 m(2), p=0·84). Hydroxycarbamide significantly decreased pain (177 events in 62 patients vs 375 events in 75 patients in the placebo group, p=0·002) and dactylitis (24 events in 14 patients vs 123 events in 42 patients in the placebo group, p<0·0001), with some evidence for decreased acute chest syndrome, hospitalisation rates, and transfusion. Hydroxyurea increased haemoglobin and fetal haemoglobin, and decreased white blood-cell count. Toxicity was limited to mild-to-moderate neutropenia.
Interpretation: On the basis of the safety and efficacy data from this trial, hydroxycarbamide can now be considered for all very young children with sickle-cell anaemia.
Funding: The US National Heart, Lung, and Blood Institute; and the National Institute of Child Health and Human Development.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors had conflicts of interest.
Figures
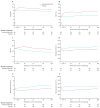
 ); the Placebo group is indicated by blue lines (
); the Placebo group is indicated by blue lines (
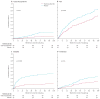
 ); the Placebo group by blue lines (
); the Placebo group by blue lines (
 ). The cumulative probability curves were generated by the time to first event for acute chest syndrome (Panel A), pain (B), dactylitis (C), and transfusion (D). The differences in the events between the treatment groups were evaluated from the log-rank life test. The p-values for cumulative probability curves for ACS, pain, dactylitis, and transfusion were 0·02, 0·002, <0·001, and 0·03, respectively.
). The cumulative probability curves were generated by the time to first event for acute chest syndrome (Panel A), pain (B), dactylitis (C), and transfusion (D). The differences in the events between the treatment groups were evaluated from the log-rank life test. The p-values for cumulative probability curves for ACS, pain, dactylitis, and transfusion were 0·02, 0·002, <0·001, and 0·03, respectively.Comment in
-
Hydroxycarbamide for sickle-cell anaemia in infancy.Lancet. 2011 May 14;377(9778):1628-30. doi: 10.1016/S0140-6736(11)60511-4. Lancet. 2011. PMID: 21571132 No abstract available.
-
The safety and efficacy of hydroxycarbamide in infants with sickle cell anemia.Expert Rev Hematol. 2011 Aug;4(4):407-9. doi: 10.1586/ehm.11.40. Expert Rev Hematol. 2011. PMID: 21801131
-
Hydroxycarbamide for very young children with sickle cell anaemia: no effect on the primary outcomes of spleen or kidney function, but evidence for decreased pain and dactylitis, with minimal toxicity.Evid Based Med. 2012 Apr;17(2):37-8. doi: 10.1136/ebm.2011.100104. Epub 2011 Sep 3. Evid Based Med. 2012. PMID: 21891796 No abstract available.
-
Hydroxycarbamide use in young children with sickle-cell anaemia.Lancet. 2011 Nov 19;378(9805):1777. doi: 10.1016/S0140-6736(11)61770-4. Lancet. 2011. PMID: 22098850 No abstract available.
-
Hydroxycarbamide use in young children with sickle-cell anaemia.Lancet. 2011 Nov 19;378(9805):1777. doi: 10.1016/S0140-6736(11)61771-6. Lancet. 2011. PMID: 22098851 No abstract available.
Similar articles
-
Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial.Lancet. 2016 Feb 13;387(10019):661-670. doi: 10.1016/S0140-6736(15)01041-7. Epub 2015 Dec 6. Lancet. 2016. PMID: 26670617 Free PMC article. Clinical Trial.
-
Hydroxyurea (hydroxycarbamide) for sickle cell disease.Cochrane Database Syst Rev. 2017 Apr 20;4(4):CD002202. doi: 10.1002/14651858.CD002202.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Sep 1;9:CD002202. doi: 10.1002/14651858.CD002202.pub3. PMID: 28426137 Free PMC article. Updated. Review.
-
Hydroxyurea (hydroxycarbamide) for sickle cell disease.Cochrane Database Syst Rev. 2022 Sep 1;9(9):CD002202. doi: 10.1002/14651858.CD002202.pub3. Cochrane Database Syst Rev. 2022. PMID: 36047926 Free PMC article. Review.
-
Hydroxyurea dose optimisation for children with sickle cell anaemia in sub-Saharan Africa (REACH): extended follow-up of a multicentre, open-label, phase 1/2 trial.Lancet Haematol. 2024 Jun;11(6):e425-e435. doi: 10.1016/S2352-3026(24)00078-4. Epub 2024 Apr 30. Lancet Haematol. 2024. PMID: 38701812 Clinical Trial.
-
Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease.Cochrane Database Syst Rev. 2020 Jul 27;7(7):CD003146. doi: 10.1002/14651858.CD003146.pub4. Cochrane Database Syst Rev. 2020. PMID: 32716555 Free PMC article.
Cited by
-
Prescribing Hydroxyurea in Sickle Cell Disease Patients: The Pattern and Association with Co-Prescribed Medications Used to Manage the Disease Complications.J Clin Med. 2024 Oct 19;13(20):6254. doi: 10.3390/jcm13206254. J Clin Med. 2024. PMID: 39458204 Free PMC article.
-
Newborn Screening for Sickle Cell Disease in Catalonia between 2015 and 2022-Epidemiology and Impact on Clinical Events.Int J Neonatal Screen. 2024 Oct 3;10(4):69. doi: 10.3390/ijns10040069. Int J Neonatal Screen. 2024. PMID: 39449357 Free PMC article.
-
Sickle Cell Disease.Transfus Med Hemother. 2024 Aug 6;51(5):332-344. doi: 10.1159/000540149. eCollection 2024 Oct. Transfus Med Hemother. 2024. PMID: 39371249 Free PMC article. Review.
-
Prevalence and associated factors of glomerular hyperfiltration among adult stable sickle cells in Kinshasa, DR Congo.Ren Fail. 2024 Dec;46(2):2407888. doi: 10.1080/0886022X.2024.2407888. Epub 2024 Sep 27. Ren Fail. 2024. PMID: 39329176 Free PMC article.
-
Fostering a healthier generation of children with sickle cell disease through advancements in care.Pediatr Res. 2024 Sep 13. doi: 10.1038/s41390-024-03566-w. Online ahead of print. Pediatr Res. 2024. PMID: 39271903 Review.
References
-
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. - PubMed
-
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. - PubMed
-
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. - PubMed
-
- Ferster A, Tahriri P, Vermylen C, et al. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97:3628–3632. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01-HB-07150/HB/NHLBI NIH HHS/United States
- N01-HB-07159/HB/NHLBI NIH HHS/United States
- N01-HB-07160/HB/NHLBI NIH HHS/United States
- N01-HB-07153/HB/NHLBI NIH HHS/United States
- N01-HB-07156/HB/NHLBI NIH HHS/United States
- N01HB07160/HB/NHLBI NIH HHS/United States
- N01-HB-07157/HB/NHLBI NIH HHS/United States
- N01-HB-07155/HB/NHLBI NIH HHS/United States
- N01-HB-07158/HB/NHLBI NIH HHS/United States
- N01 HB007155/HB/NHLBI NIH HHS/United States
- N01HB07150/HB/NHLBI NIH HHS/United States
- N01-HB-07152/HB/NHLBI NIH HHS/United States
- N01-HB-07151/HB/NHLBI NIH HHS/United States
- N01-HB-07154/HB/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

