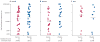Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study
- PMID: 21570355
- PMCID: PMC3417349
- DOI: 10.1016/S1473-3099(11)70098-6
Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study
Erratum in
- Lancet Infect Dis. 2011 Jul;11(7):495
Abstract
Background: The MRKAd5 HIV-1 gag/pol/nef subtype B vaccine was designed to elicit T-cell-mediated immune responses capable of providing complete or partial protection from HIV-1 infection or a decrease in viral load after acquisition. We aim to assess the safety and efficacy of the vaccine in South Africa, where the major circulating clade is subtype C.
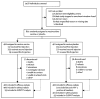
Methods: We did a phase 2b double-blind, randomised test-of-concept study in sexually active HIV-1 seronegative participants at five sites in South Africa. Randomisation was by a computer-generated random number sequence. The vaccine and placebo were given by intramuscular injection on a 0, 1, 6 month schedule. Our coprimary endpoints were a vaccine-induced reduction in HIV-1 acquisition and viral-load setpoint. These endpoints were assessed independently in the modified intention-to-treat (MITT) cohort with two-tailed significance tests stratified by sex. We assessed immunogenicity by interferon-γ ELISPOT in peripheral-blood mononuclear cells. After the lack of efficacy of the MRKAd5 HIV-1 vaccine in the Step study, enrolment and vaccination in our study was halted, treatment allocations were unmasked, and follow-up continued. This study is registered with the South Africa National Health Research Database, number DOH-27-0207-1539, and ClinicalTrials.gov, number NCT00413725.
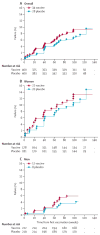
Findings: 801 of a scheduled 3000 participants, of whom 360 (45%) were women, were randomly assigned to receive either vaccine or placebo. 445 participants (56%) had adenovirus serotype 5 (Ad5) titres greater than 200, and 129 men (29%) were circumcised. 34 MITT participants in the vaccine group were diagnosed with HIV-1 (incidence rate 4·54 per 100 person-years) and 28 in the placebo group (3·70 per 100 person-years). There was no evidence of vaccine efficacy; the hazard ratio adjusted for sex was 1·25 (95% CI 0·76-2·05). Vaccine efficacy did not differ by Ad5 titre, sex, age, herpes simplex virus type 2 status, or circumcision. The geometric mean viral-load setpoint was 20,483 copies per mL (n=33) in the vaccine group and 34,032 copies per mL (n=28) in the placebo group (p=0·39). The vaccine elicited interferon-γ-secreting T cells that recognised both clade B (89%) and C (77%) antigens.
Interpretation: The MRKAd5 HIV-1 vaccine did not prevent HIV-1 infection or lower viral-load setpoint; however, stopping our trial early probably compromised our ability to draw conclusions. The high incidence rates noted in South Africa highlight the crucial need for intensified efforts to develop an efficacious vaccine.
Funding: The US National Institute of Allergy and Infectious Disease and Merck and Co Inc.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Comment in
-
The HVTN 503/Phambili study: efficacy is always the issue.Lancet Infect Dis. 2011 Jul;11(7):490-1. doi: 10.1016/S1473-3099(11)70103-7. Epub 2011 May 11. Lancet Infect Dis. 2011. PMID: 21570356 No abstract available.
-
Immunology. Immune activation with HIV vaccines.Science. 2014 Apr 4;344(6179):49-51. doi: 10.1126/science.1250672. Science. 2014. PMID: 24700849 Free PMC article. No abstract available.
Similar articles
-
Recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: unblinded, long-term follow-up of the phase 2b HVTN 503/Phambili study.Lancet Infect Dis. 2014 May;14(5):388-96. doi: 10.1016/S1473-3099(14)70020-9. Epub 2014 Feb 20. Lancet Infect Dis. 2014. PMID: 24560541 Free PMC article. Clinical Trial.
-
Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial.Lancet. 2008 Nov 29;372(9653):1881-1893. doi: 10.1016/S0140-6736(08)61591-3. Epub 2008 Nov 13. Lancet. 2008. PMID: 19012954 Free PMC article. Clinical Trial.
-
Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial.Lancet HIV. 2018 Jul;5(7):e366-e378. doi: 10.1016/S2352-3018(18)30071-7. Epub 2018 Jun 18. Lancet HIV. 2018. PMID: 29898870 Free PMC article. Clinical Trial.
-
Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine.Curr Opin HIV AIDS. 2010 Sep;5(5):357-61. doi: 10.1097/COH.0b013e32833d2d2b. Curr Opin HIV AIDS. 2010. PMID: 20978374 Free PMC article. Review.
-
A tale of four studies: HIV vaccine immunogenicity and efficacy in clinical trials.Lancet HIV. 2021 Jul;8(7):e449-e452. doi: 10.1016/S2352-3018(21)00073-4. Epub 2021 May 21. Lancet HIV. 2021. PMID: 34029515 Free PMC article. Review.
Cited by
-
Loss of HIV candidate vaccine efficacy in male macaques by mucosal nanoparticle immunization rescued by V2-specific response.Nat Commun. 2024 Oct 22;15(1):9102. doi: 10.1038/s41467-024-53359-2. Nat Commun. 2024. PMID: 39438480 Free PMC article.
-
A Zigzag but Upward Way to Develop an HIV-1 Vaccine.Vaccines (Basel). 2020 Sep 8;8(3):511. doi: 10.3390/vaccines8030511. Vaccines (Basel). 2020. PMID: 32911701 Free PMC article. Review.
-
Testing the waters: Ethical considerations for including PrEP in a phase IIb HIV vaccine efficacy trial.Clin Trials. 2015 Aug;12(4):394-402. doi: 10.1177/1740774515579165. Epub 2015 Apr 7. Clin Trials. 2015. PMID: 25851992 Free PMC article.
-
Vaccine protection against rectal acquisition of SIVmac239 in rhesus macaques.PLoS Pathog. 2019 Sep 30;15(9):e1008015. doi: 10.1371/journal.ppat.1008015. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31568531 Free PMC article.
-
Safety and immunogenicity of a multivalent HIV vaccine comprising envelope protein with either DNA or NYVAC vectors (HVTN 096): a phase 1b, double-blind, placebo-controlled trial.Lancet HIV. 2019 Nov;6(11):e737-e749. doi: 10.1016/S2352-3018(19)30262-0. Epub 2019 Oct 7. Lancet HIV. 2019. PMID: 31601541 Free PMC article. Clinical Trial.
References
-
- UNAIDS. Report on the global AIDS epidemic. Geneva, CH: Joint United Nations Programme on HIV/AIDS; 2008.
-
- Shisana O, Rehle T, Simbayi L, Zuma K, Jooste S, Pillay-van-Wyk V, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenagers? Cape Town: HSRC Press; 2009.
-
- Harrer T, Harrer E, Kalams SA, Elbeik T, Staprans SI, Feinberg MB, et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12(7):585–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical