Identification of a novel temperature sensitive promoter in CHO cells
- PMID: 21569433
- PMCID: PMC3118111
- DOI: 10.1186/1472-6750-11-51
Identification of a novel temperature sensitive promoter in CHO cells
Abstract
Background: The Chinese hamster ovary (CHO) expression system is the leading production platform for manufacturing biopharmaceuticals for the treatment of numerous human diseases. Efforts to optimize the production process also include the genetic construct encoding the therapeutic gene. Here we report about the successful identification of an endogenous highly active gene promoter obtained from CHO cells which shows conditionally inducible gene expression at reduced temperature.
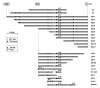
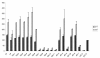
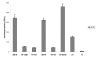
Results: Based on CHO microarray expression data abundantly transcribed genes were selected as potential promoter candidates. The S100a6 (calcyclin) and its flanking regions were identified from a genomic CHO-K1 lambda-phage library. Computational analyses showed a predicted TSS, a TATA-box and several TFBSs within the 1.5 kb region upstream the ATG start signal. Various constructs were investigated for promoter activity at 37°C and 33°C in transient luciferase reporter gene assays. Most constructs showed expression levels even higher than the SV40 control and on average a more than two-fold increase at lower temperature. We identified the core promoter sequence (222 bp) comprising two SP1 sites and could show a further increase in activity by duplication of this minimal sequence.
Conclusions: This novel CHO promoter permits conditionally high-level gene expression. Upon a shift to 33°C, a two to three-fold increase of basal productivity (already higher than SV40 promoter) is achieved. This property is of particular advantage for a process with reduced expression during initial cell growth followed by the production phase at low temperature with a boost in expression. Additionally, production of toxic proteins becomes feasible, since cell metabolism and gene expression do not directly interfere. The CHO S100a6 promoter can be characterized as cold-shock responsive with the potential for improving process performance of mammalian expression systems.
Figures
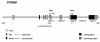


Similar articles
-
Identification of a novel enhancer that binds Sp1 and contributes to induction of cold-inducible RNA-binding protein (cirp) expression in mammalian cells.BMC Biotechnol. 2012 Oct 10;12:72. doi: 10.1186/1472-6750-12-72. BMC Biotechnol. 2012. PMID: 23046908 Free PMC article.
-
Bioinformatic Identification of Chinese Hamster Ovary (CHO) Cold-Shock Genes and Biological Evidence of their Cold-Inducible Promoters.Biotechnol J. 2020 Mar;15(3):e1900359. doi: 10.1002/biot.201900359. Epub 2019 Dec 11. Biotechnol J. 2020. PMID: 31785035
-
Cloning of the murine Drm gene (Cktsf1b1) and characterization of its oncogene suppressible promoter.Cytogenet Cell Genet. 2000;89(3-4):242-51. doi: 10.1159/000015625. Cytogenet Cell Genet. 2000. PMID: 10965135
-
Manipulating gene expression levels in mammalian cell factories: An outline of synthetic molecular toolboxes to achieve multiplexed control.N Biotechnol. 2024 Mar 25;79:1-19. doi: 10.1016/j.nbt.2023.11.003. Epub 2023 Nov 30. N Biotechnol. 2024. PMID: 38040288 Review.
-
The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production.Biotechnol Bioeng. 2006 Apr 5;93(5):829-35. doi: 10.1002/bit.20789. Biotechnol Bioeng. 2006. PMID: 16329142 Review.
Cited by
-
Enhancing productivity of Chinese hamster ovary (CHO) cells: synergistic strategies combining low-temperature culture and mTORC1 signaling engineering.Front Bioeng Biotechnol. 2023 Nov 21;11:1268048. doi: 10.3389/fbioe.2023.1268048. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38076428 Free PMC article.
-
Study of the regulatory elements of the Ovalbumin gene promoter using CRISPR technology in chicken cells.J Biol Eng. 2023 Jul 17;17(1):46. doi: 10.1186/s13036-023-00367-3. J Biol Eng. 2023. PMID: 37461059 Free PMC article.
-
Synthetic enhancers including TFREs improve transgene expression in CHO cells.Heliyon. 2024 Feb 29;10(5):e26901. doi: 10.1016/j.heliyon.2024.e26901. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38468921 Free PMC article.
-
The potential of cold-shock promoters for the expression of recombinant proteins in microbes and mammalian cells.J Genet Eng Biotechnol. 2022 Dec 29;20(1):173. doi: 10.1186/s43141-022-00455-9. J Genet Eng Biotechnol. 2022. PMID: 36580173 Free PMC article. Review.
-
Alternative promoters regulate cold inducible RNA-binding (CIRP) gene expression and enhance transgene expression in mammalian cells.Mol Biotechnol. 2013 Jun;54(2):238-49. doi: 10.1007/s12033-013-9649-5. Mol Biotechnol. 2013. PMID: 23589278
References
-
- Mohan C, Kim YG, Koo J, Lee GM. Assessment of cell engineering strategies for improved therapeutic protein production in CHO cells. Biotechnol J. 2008;6(5):624–630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

