Cockayne syndrome B protein antagonizes OGG1 in modulating CAG repeat length in vivo
- PMID: 21566259
- PMCID: PMC3156601
- DOI: 10.18632/aging.100324
Cockayne syndrome B protein antagonizes OGG1 in modulating CAG repeat length in vivo
Abstract
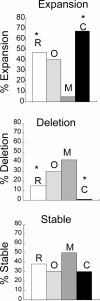
OGG1 and MSH2/MSH3 promote CAG repeat expansion at Huntington's disease (HD) locusin vivo during removal of oxidized bases from DNA. CSB, a transcription-coupled repair (TCR) protein, facilitates repair of some of the same oxidative lesions. In vitro, a knock down CSB results in a reduction of transcription-induced deletions at CAG repeat tract. To test the role of CSB in vivo, we measured intergenerational and somatic expansion of CAG tracts in HD mice lacking CSB, OGG1, or both. We provide evidence that CSB protects CAG repeats from expansion by either active reduction of the tract length during parent-child transmission, or by antagonizing the action of OGG1, which tends to promote expansion in somatic cells. These results raise a possibility that actions of transcription-coupled and base excision repair pathways lead to different outcomes at CAG tracts in vivo.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures

Similar articles
-
Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington's disease transgenic mice.PLoS Genet. 2009 Dec;5(12):e1000749. doi: 10.1371/journal.pgen.1000749. Epub 2009 Dec 4. PLoS Genet. 2009. PMID: 19997493 Free PMC article.
-
Poly(ADP-ribose) polymerase 1 (PARP1) promotes oxidative stress-induced association of Cockayne syndrome group B protein with chromatin.J Biol Chem. 2018 Nov 16;293(46):17863-17874. doi: 10.1074/jbc.RA118.004548. Epub 2018 Sep 28. J Biol Chem. 2018. PMID: 30266807 Free PMC article.
-
Cockayne syndrome group B protein stimulates repair of formamidopyrimidines by NEIL1 DNA glycosylase.J Biol Chem. 2009 Apr 3;284(14):9270-9. doi: 10.1074/jbc.M807006200. Epub 2009 Jan 29. J Biol Chem. 2009. PMID: 19179336 Free PMC article.
-
The role of Cockayne Syndrome group B (CSB) protein in base excision repair and aging.Mech Ageing Dev. 2008 Jul-Aug;129(7-8):441-8. doi: 10.1016/j.mad.2008.04.009. Epub 2008 Apr 30. Mech Ageing Dev. 2008. PMID: 18541289 Free PMC article. Review.
-
Methods for Assessing DNA Repair and Repeat Expansion in Huntington's Disease.Methods Mol Biol. 2018;1780:483-495. doi: 10.1007/978-1-4939-7825-0_22. Methods Mol Biol. 2018. PMID: 29856032 Review.
Cited by
-
MSH3 polymorphisms and protein levels affect CAG repeat instability in Huntington's disease mice.PLoS Genet. 2013;9(2):e1003280. doi: 10.1371/journal.pgen.1003280. Epub 2013 Feb 28. PLoS Genet. 2013. PMID: 23468640 Free PMC article.
-
Abnormal base excision repair at trinucleotide repeats associated with diseases: a tissue-selective mechanism.Genes (Basel). 2013 Jul 25;4(3):375-87. doi: 10.3390/genes4030375. Genes (Basel). 2013. PMID: 24705210 Free PMC article.
-
Neil1 is a genetic modifier of somatic and germline CAG trinucleotide repeat instability in R6/1 mice.Hum Mol Genet. 2012 Nov 15;21(22):4939-47. doi: 10.1093/hmg/dds337. Epub 2012 Aug 21. Hum Mol Genet. 2012. PMID: 22914735 Free PMC article.
-
Repeat instability during DNA repair: Insights from model systems.Crit Rev Biochem Mol Biol. 2015 Mar-Apr;50(2):142-67. doi: 10.3109/10409238.2014.999192. Epub 2015 Jan 22. Crit Rev Biochem Mol Biol. 2015. PMID: 25608779 Free PMC article. Review.
-
Xpa deficiency reduces CAG trinucleotide repeat instability in neuronal tissues in a mouse model of SCA1.Hum Mol Genet. 2011 Dec 15;20(24):4822-30. doi: 10.1093/hmg/ddr421. Epub 2011 Sep 15. Hum Mol Genet. 2011. PMID: 21926083 Free PMC article.
References
-
- Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447:932–940. - PubMed
-
- Pearson CE, Edamura NK, Cleary JD. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet. 2005;6:729–742. - PubMed
-
- Kovtun IV, McMurray CT. Features of trinucleotide repeat instability in vivo. Cell Res. 2008;18:198–213. - PubMed
-
- Dragileva E, Hendricks A, Teed A, Gillis T, Lopez ET, Friedberg EC, Kucherlapati R, Edelmann W, Lunetta KL, MacDonald ME, Wheeler VC. Intergenerational and striatal CAG repeat instability in Huntington's disease knock-in mice involve different DNA repair genes. Neurobiol Dis. 2009;2009;33:37–47. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

