In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins
- PMID: 21562231
- PMCID: PMC3305909
- DOI: 10.1126/scitranslmed.3002376
In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins
Abstract
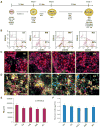
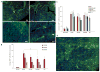
Human induced pluripotent stem cells (iPSCs) are a potential source of hepatocytes for liver transplantation to treat end-stage liver disease. In vitro differentiation of human iPSCs into hepatic cells has been achieved using a multistage differentiation protocol, but whether these cells are functional and capable of engrafting and regenerating diseased liver tissue is not clear. We show that human iPSC-derived hepatic cells at various differentiation stages can engraft the liver in a mouse transplantation model. Using the same differentiation and transplantation protocols, we also assessed the ability of human iPSCs derived from each of the three developmental germ layer tissues (that is, ectoderm, mesoderm, and endoderm) to regenerate mouse liver. These iPSC lines, with similar but distinct global DNA methylation patterns, differentiated into multistage hepatic cells with an efficiency similar to that of human embryonic stem cells. Human hepatic cells at various differentiation stages derived from iPSC lines of different origins successfully repopulated the liver tissue of mice with liver cirrhosis. They also secreted human-specific liver proteins into mouse blood at concentrations comparable to that of proteins secreted by human primary hepatocytes. Our results demonstrate the engraftment and liver regenerative capabilities of human iPSC-derived multistage hepatic cells in vivo and suggest that human iPSCs of distinct origins and regardless of their parental epigenetic memory can efficiently differentiate along the hepatic lineage.
Conflict of interest statement
Figures


Similar articles
-
Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells.J Am Coll Cardiol. 2014 Aug 5;64(5):436-48. doi: 10.1016/j.jacc.2014.04.056. J Am Coll Cardiol. 2014. PMID: 25082575 Free PMC article.
-
Early prediction of the differentiation potential during the formation of human iPSC-derived embryoid bodies.Biochem Biophys Res Commun. 2019 Aug 27;516(3):673-679. doi: 10.1016/j.bbrc.2019.06.081. Epub 2019 Jun 24. Biochem Biophys Res Commun. 2019. PMID: 31248595
-
Efficient hematopoietic redifferentiation of induced pluripotent stem cells derived from primitive murine bone marrow cells.Stem Cells Dev. 2012 Mar 20;21(5):689-701. doi: 10.1089/scd.2011.0010. Epub 2011 Aug 24. Stem Cells Dev. 2012. PMID: 21732815
-
Induced pluripotent stem cells and hepatic differentiation.J Chin Med Assoc. 2013 Nov;76(11):599-605. doi: 10.1016/j.jcma.2013.07.007. Epub 2013 Aug 9. J Chin Med Assoc. 2013. PMID: 23933345 Review.
-
Thinking outside the liver: induced pluripotent stem cells for hepatic applications.World J Gastroenterol. 2013 Jun 14;19(22):3385-96. doi: 10.3748/wjg.v19.i22.3385. World J Gastroenterol. 2013. PMID: 23801830 Free PMC article. Review.
Cited by
-
Translation of Human-Induced Pluripotent Stem Cells: From Clinical Trial in a Dish to Precision Medicine.J Am Coll Cardiol. 2016 May 10;67(18):2161-2176. doi: 10.1016/j.jacc.2016.01.083. J Am Coll Cardiol. 2016. PMID: 27151349 Free PMC article. Review.
-
Stem cell differentiation and human liver disease.World J Gastroenterol. 2012 May 7;18(17):2018-25. doi: 10.3748/wjg.v18.i17.2018. World J Gastroenterol. 2012. PMID: 22563188 Free PMC article. Review.
-
Current status of hepatocyte-like cell therapy from stem cells.Surg Today. 2021 Mar;51(3):340-349. doi: 10.1007/s00595-020-02092-6. Epub 2020 Aug 4. Surg Today. 2021. PMID: 32754843 Review.
-
Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells.Blood. 2011 Aug 18;118(7):1801-5. doi: 10.1182/blood-2011-03-340620. Epub 2011 May 31. Blood. 2011. PMID: 21628406 Free PMC article.
-
Human induced pluripotent cells resemble embryonic stem cells demonstrating enhanced levels of DNA repair and efficacy of nonhomologous end-joining.Mutat Res. 2011 Aug 1;713(1-2):8-17. doi: 10.1016/j.mrfmmm.2011.05.018. Epub 2011 Jun 28. Mutat Res. 2011. PMID: 21718709 Free PMC article.
References
-
- Locke JE, Sun Z, Warren DS, Sheets TP, Holzer H, Shamblott MJ, Montgomery RA, Cameron AM. Generation of humanized animal livers using embryoid body-derived stem cell transplant. Ann Surg. 2008;248:487–493. - PubMed
-
- Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840–2850. - PubMed
-
- Lázaro CA, Rhim JA, Yamada Y, Fausto N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 1998;58:5514–5522. - PubMed
-
- Czyz J, Wiese C, Rolletschek A, Blyszczuk P, Cross M, Wobus AM. Potential of embryonic and adult stem cells in vitro. Biol Chem. 2003;384:1391–1409. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

