Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection
- PMID: 21549619
- PMCID: PMC3103642
- DOI: 10.1016/j.immuni.2011.03.020
Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection
Abstract
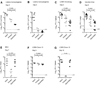
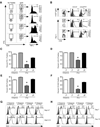
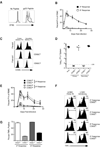
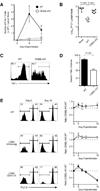
Infection or vaccination confers heightened resistance to pathogen rechallenge because of quantitative and qualitative differences between naive and primary memory T cells. Herein, we show that secondary (boosted) memory CD8+ T cells were better than primary memory CD8+ T cells in controlling some, but not all acute infections with diverse pathogens. However, secondary memory CD8+ T cells were less efficient than an equal number of primary memory cells at preventing chronic LCMV infection and are more susceptible to functional exhaustion. Importantly, localization of memory CD8+ T cells within lymph nodes, which is reduced by antigen restimulation, was critical for both viral control in lymph nodes and for the sustained CD8+ T cell response required to prevent chronic LCMV infection. Thus, repeated antigen stimulation shapes memory CD8+ T cell populations to either enhance or decrease per cell protective immunity in a pathogen-specific manner, a concept of importance in vaccine design against specific diseases.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Gammaherpesvirus latency differentially impacts the generation of primary versus secondary memory CD8+ T cells during subsequent infection.J Virol. 2014 Nov;88(21):12740-51. doi: 10.1128/JVI.02106-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142586 Free PMC article.
-
The initial draining lymph node primes the bulk of the CD8 T cell response and influences memory T cell trafficking after a systemic viral infection.PLoS Pathog. 2012;8(12):e1003054. doi: 10.1371/journal.ppat.1003054. Epub 2012 Dec 6. PLoS Pathog. 2012. PMID: 23236277 Free PMC article.
-
Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection.PLoS Pathog. 2015 Oct 20;11(10):e1005219. doi: 10.1371/journal.ppat.1005219. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26485703 Free PMC article.
-
Memory CD8⁺ T cell protection.Adv Exp Med Biol. 2013;785:77-86. doi: 10.1007/978-1-4614-6217-0_9. Adv Exp Med Biol. 2013. PMID: 23456840 Review.
-
Influence of time and number of antigen encounters on memory CD8 T cell development.Immunol Res. 2014 Aug;59(1-3):35-44. doi: 10.1007/s12026-014-8522-3. Immunol Res. 2014. PMID: 24825776 Review.
Cited by
-
Antigen experience shapes phenotype and function of memory Th1 cells.PLoS One. 2013 Jun 7;8(6):e65234. doi: 10.1371/journal.pone.0065234. Print 2013. PLoS One. 2013. PMID: 23762323 Free PMC article.
-
Discriminating Protective from Nonprotective Plasmodium-Specific CD8+ T Cell Responses.J Immunol. 2016 May 15;196(10):4253-62. doi: 10.4049/jimmunol.1600155. Epub 2016 Apr 15. J Immunol. 2016. PMID: 27084099 Free PMC article.
-
Differential Immune Response Following Intranasal and Intradermal Infection with Francisella tularensis: Implications for Vaccine Development.Microorganisms. 2021 Apr 30;9(5):973. doi: 10.3390/microorganisms9050973. Microorganisms. 2021. PMID: 33946283 Free PMC article. Review.
-
Antigen-dependent and -independent contributions to primary memory CD8 T cell activation and protection following infection.Sci Rep. 2015 Dec 10;5:18022. doi: 10.1038/srep18022. Sci Rep. 2015. PMID: 26658291 Free PMC article.
-
Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8+ T cells.Sci Immunol. 2017 Oct 13;2(16):eaan6049. doi: 10.1126/sciimmunol.aan6049. Sci Immunol. 2017. PMID: 29030501 Free PMC article.
References
-
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. - PubMed
-
- Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305:205–208. - PubMed
-
- Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. - PubMed
-
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. - PubMed
-
- Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI050073/AI/NIAID NIH HHS/United States
- R01 AI059752-05/AI/NIAID NIH HHS/United States
- R01 AI059752/AI/NIAID NIH HHS/United States
- AI59752/AI/NIAID NIH HHS/United States
- R01 AI042767/AI/NIAID NIH HHS/United States
- AI42767/AI/NIAID NIH HHS/United States
- AI50073/AI/NIAID NIH HHS/United States
- R01 AI046653-10/AI/NIAID NIH HHS/United States
- R01 AI050073-10/AI/NIAID NIH HHS/United States
- R01 AI046653/AI/NIAID NIH HHS/United States
- R37 AI042767/AI/NIAID NIH HHS/United States
- R37 AI042767-14/AI/NIAID NIH HHS/United States
- R21 AI042767/AI/NIAID NIH HHS/United States
- AI46653/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

