Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice
- PMID: 21543771
- PMCID: PMC3142254
- DOI: 10.1095/biolreprod.110.090621
Sertoli cell-specific deletion of the androgen receptor compromises testicular immune privilege in mice
Abstract
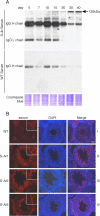
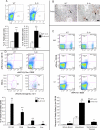
In the mammalian testis, meiotic and postmeiotic germ cell antigens are granted immune privilege. Both local immune suppression and specialized intercellular junctions between somatic Sertoli cells have been proposed to contribute to a highly restricted and effective blood-testis barrier (BTB) that helps maintain tolerance to germ cell antigens. Several studies have suggested that androgens play a role in immune suppression, although direct evidence for this is lacking. We previously reported that Sertoli cell-specific ablation of the androgen receptor (Ar) decreases expression of Cldn3, an androgen-regulated gene and component of Sertoli cell tight junctions, and increases the permeability of the BTB to biotin, a small-molecular-weight tracer. The physiological consequences of Sertoli cell-specific Ar (S-Ar) ablation on immune privilege are unknown. Here we show that in the testes of S-Ar mutant mice, the ultrastructure of Sertoli cell tight junctions is defective and testicular IgG levels are elevated. The interstitium of S-Ar mutant testes becomes populated with macrophages, neutrophils, plasma cells, and eosinophils, and serum samples of mutant mice contain antibodies against germ cell antigens. Together, these results suggest that Sertoli cell-specific deletion of the androgen receptor results in loss of testicular immune privilege. Suppressed levels of androgen signaling may be a contributing factor in idiopathic male infertility.
Figures


Similar articles
-
Establishment, maintenance and functional integrity of the blood-testis barrier in organotypic cultures of fresh and frozen/thawed prepubertal mouse testes.Mol Hum Reprod. 2017 May 1;23(5):304-320. doi: 10.1093/molehr/gax017. Mol Hum Reprod. 2017. PMID: 28333312
-
Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model.J Cell Physiol. 2005 Oct;205(1):141-57. doi: 10.1002/jcp.20377. J Cell Physiol. 2005. PMID: 15880438
-
Temporal role of Sertoli cell androgen receptor expression in spermatogenic development.Mol Endocrinol. 2013 Jan;27(1):12-24. doi: 10.1210/me.2012-1219. Epub 2012 Nov 16. Mol Endocrinol. 2013. PMID: 23160479 Free PMC article.
-
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice.Endocr Rev. 2009 Apr;30(2):119-32. doi: 10.1210/er.2008-0025. Epub 2009 Jan 27. Endocr Rev. 2009. PMID: 19176467 Free PMC article. Review.
-
ETV5 is required for continuous spermatogenesis in adult mice and may mediate blood testes barrier function and testicular immune privilege.Ann N Y Acad Sci. 2007 Dec;1120:144-51. doi: 10.1196/annals.1411.005. Epub 2007 Oct 2. Ann N Y Acad Sci. 2007. PMID: 17911411 Free PMC article. Review.
Cited by
-
Testosterone signaling and the regulation of spermatogenesis.Spermatogenesis. 2011 Apr;1(2):116-120. doi: 10.4161/spmg.1.2.16956. Spermatogenesis. 2011. PMID: 22319659 Free PMC article.
-
The Sertoli cell: one hundred fifty years of beauty and plasticity.Andrology. 2016 Mar;4(2):189-212. doi: 10.1111/andr.12165. Epub 2016 Feb 4. Andrology. 2016. PMID: 26846984 Free PMC article. Review.
-
Effect of extracellular matrix on testosterone production during in vitro culture of bovine testicular cells.Vet Res Forum. 2017 Winter;8(1):7-13. Epub 2017 Mar 15. Vet Res Forum. 2017. PMID: 28473891 Free PMC article.
-
Transcription factor Dmrt1 triggers the SPRY1-NF-κB pathway to maintain testicular immune homeostasis and male fertility.Zool Res. 2023 May 18;44(3):505-521. doi: 10.24272/j.issn.2095-8137.2022.440. Zool Res. 2023. PMID: 37070575 Free PMC article.
-
Sertoli cells--immunological sentinels of spermatogenesis.Semin Cell Dev Biol. 2014 Jun;30:36-44. doi: 10.1016/j.semcdb.2014.02.011. Epub 2014 Mar 3. Semin Cell Dev Biol. 2014. PMID: 24603046 Free PMC article. Review.
References
-
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol 2011; 335: 60 68. - PubMed
-
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell 1987; 49: 273 280. - PubMed
-
- Nossal GJ. Negative selection of lymphocytes. Cell 1994; 76: 229 239. - PubMed
-
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3: 308 326. - PubMed
-
- Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Rec 1977; 187: 347 366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

