A functional role for Nlrp6 in intestinal inflammation and tumorigenesis
- PMID: 21543645
- PMCID: PMC3133458
- DOI: 10.4049/jimmunol.1100412
A functional role for Nlrp6 in intestinal inflammation and tumorigenesis
Abstract
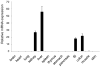
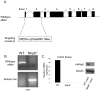
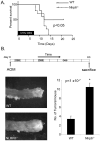
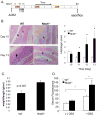
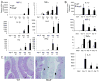
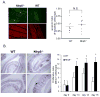
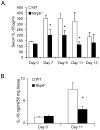
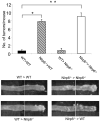
The nucleotide-binding oligomerization domain-like receptor (NLR) family member, Nlrp6, has been implicated in inflammasome signaling to activate caspase-1, which is essential for the production of mature IL-1β and IL-18. However, a function for Nlrp6 in vivo has never been demonstrated. Due to the relative high expression of Nlrp6 in intestinal tissue, we hypothesized that Nlrp6 has a role in intestinal homeostasis. Indeed, Nlrp6-deficient mice are more susceptible to chemically induced colitis as well as colitis-induced tumorigenesis than wild-type (WT) mice. Nlrp6-deficient mice exhibited significantly more inflammation within the colon than WT mice after dextran sulfate sodium treatment. Their inability to resolve inflammation and repair damaged epithelium as efficiently as WT mice resulted in prolonged increases in epithelial proliferative activity that likely underlie the increased propensity for tumors in these mice during chronic inflammation. We further show that the activity of Nlrp6 in hematopoietic cells is critical for protection against inflammation-related colon tumorigenesis. This study highlights the importance of NLR function in maintaining intestinal homeostasis to prevent the development of aberrant inflammation and tumor development within the colon.
Figures
Similar articles
-
NLRP6 function in inflammatory monocytes reduces susceptibility to chemically induced intestinal injury.Mucosal Immunol. 2017 Mar;10(2):434-445. doi: 10.1038/mi.2016.55. Epub 2016 Jun 29. Mucosal Immunol. 2017. PMID: 27353251 Free PMC article.
-
The NLR Protein NLRP6 Does Not Impact Gut Microbiota Composition.Cell Rep. 2017 Dec 26;21(13):3653-3661. doi: 10.1016/j.celrep.2017.12.026. Cell Rep. 2017. PMID: 29281815
-
Is NLRP3 or NLRP6 inflammasome activation associated with inflammation-related lung tumorigenesis induced by benzo(a)pyrene and lipopolysaccharide?Ecotoxicol Environ Saf. 2019 Dec 15;185:109687. doi: 10.1016/j.ecoenv.2019.109687. Epub 2019 Sep 24. Ecotoxicol Environ Saf. 2019. PMID: 31561077
-
The NLRP6 inflammasome.Immunology. 2021 Mar;162(3):281-289. doi: 10.1111/imm.13293. Epub 2020 Dec 27. Immunology. 2021. PMID: 33314083 Free PMC article. Review.
-
The NLRP6 inflammasome in health and disease.Mucosal Immunol. 2020 May;13(3):388-398. doi: 10.1038/s41385-020-0256-z. Epub 2020 Jan 27. Mucosal Immunol. 2020. PMID: 31988468 Free PMC article. Review.
Cited by
-
Genetic loss of murine pyrin, the Familial Mediterranean Fever protein, increases interleukin-1β levels.PLoS One. 2012;7(11):e51105. doi: 10.1371/journal.pone.0051105. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226472 Free PMC article.
-
Inflammasome in intestinal inflammation and cancer.Mediators Inflamm. 2013;2013:654963. doi: 10.1155/2013/654963. Epub 2013 Mar 28. Mediators Inflamm. 2013. PMID: 23606794 Free PMC article. Review.
-
NLRP6 deficiency expands a novel CD103+ B cell population that confers immune tolerance in NOD mice.Front Immunol. 2023 Feb 23;14:1147925. doi: 10.3389/fimmu.2023.1147925. eCollection 2023. Front Immunol. 2023. PMID: 36911699 Free PMC article.
-
Roles of the inflammasome in the gut‑liver axis (Review).Mol Med Rep. 2019 Jan;19(1):3-14. doi: 10.3892/mmr.2018.9679. Epub 2018 Nov 20. Mol Med Rep. 2019. PMID: 30483776 Free PMC article. Review.
-
Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis.Eur J Immunol. 2014 Feb;44(2):321-7. doi: 10.1002/eji.201344135. Epub 2014 Jan 16. Eur J Immunol. 2014. PMID: 24338634 Free PMC article. Review.
References
-
- Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. - PubMed
-
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

