TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase
- PMID: 21536674
- PMCID: PMC3123070
- DOI: 10.1074/jbc.M111.225870
TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase
Abstract
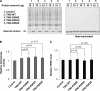
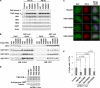
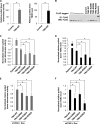
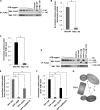
Dyskeratosis congenita (DC) is a progressive and heterogeneous congenital disorder that affects multiple systems and is characterized by bone marrow failure and a triad of abnormal skin pigmentation, nail dystrophy, and oral leukoplakia. One common feature for all DC patients is abnormally short telomeres and defects in telomere biology. Most of the known DC mutations have been found to affect core components of the telomerase holoenzyme. Recently, multiple mutations in the gene encoding the telomeric protein TIN2 have been identified in DC patients with intact telomerase genes, but the molecular mechanisms underlying TIN2 mutation-mediated DC remain unknown. Here, we demonstrate that ectopic expression of TIN2 with DC missense mutations in human cells led to accelerated telomere shortening, similar to the telomere phenotypes found in DC patients. However, this telomere shortening was not accompanied by changes in total telomerase activity, localization of TIN2, or telomere end protection status. Interestingly, we found TIN2 to participate in the TPP1-dependent recruitment of telomerase activity. Furthermore, DC mutations in TIN2 led to its decreased ability to associate with TERC and telomerase activity. Taken together, our data suggest that TIN2 mutations in DC may compromise the telomere recruitment of telomerase, leading to telomere shortening and the associated pathogenesis.
Figures

Similar articles
-
A TIN2 dyskeratosis congenita mutation causes telomerase-independent telomere shortening in mice.Genes Dev. 2014 Jan 15;28(2):153-66. doi: 10.1101/gad.233395.113. Genes Dev. 2014. PMID: 24449270 Free PMC article.
-
Hoyeraal-Hreidarsson syndrome caused by a germline mutation in the TEL patch of the telomere protein TPP1.Genes Dev. 2014 Oct 1;28(19):2090-102. doi: 10.1101/gad.248567.114. Epub 2014 Sep 18. Genes Dev. 2014. PMID: 25233904 Free PMC article.
-
The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres.PLoS Genet. 2015 Jul 31;11(7):e1005410. doi: 10.1371/journal.pgen.1005410. eCollection 2015 Jul. PLoS Genet. 2015. PMID: 26230315 Free PMC article.
-
Dyskeratosis congenita, stem cells and telomeres.Biochim Biophys Acta. 2009 Apr;1792(4):371-9. doi: 10.1016/j.bbadis.2009.01.010. Epub 2009 Feb 7. Biochim Biophys Acta. 2009. PMID: 19419704 Free PMC article. Review.
-
Short telomeres: from dyskeratosis congenita to sporadic aplastic anemia and malignancy.Transl Res. 2013 Dec;162(6):353-63. doi: 10.1016/j.trsl.2013.05.003. Epub 2013 Jun 1. Transl Res. 2013. PMID: 23732052 Free PMC article. Review.
Cited by
-
Telomerase Regulation from Beginning to the End.Genes (Basel). 2016 Sep 14;7(9):64. doi: 10.3390/genes7090064. Genes (Basel). 2016. PMID: 27649246 Free PMC article. Review.
-
The role of telomere-binding modulators in pluripotent stem cells.Protein Cell. 2020 Jan;11(1):60-70. doi: 10.1007/s13238-019-0651-y. Epub 2019 Jul 27. Protein Cell. 2020. PMID: 31350723 Free PMC article. Review.
-
A TIN2 dyskeratosis congenita mutation causes telomerase-independent telomere shortening in mice.Genes Dev. 2014 Jan 15;28(2):153-66. doi: 10.1101/gad.233395.113. Genes Dev. 2014. PMID: 24449270 Free PMC article.
-
Structural Features of Nucleoprotein CST/Shelterin Complex Involved in the Telomere Maintenance and Its Association with Disease Mutations.Cells. 2020 Feb 4;9(2):359. doi: 10.3390/cells9020359. Cells. 2020. PMID: 32033110 Free PMC article. Review.
-
Cold-inducible RNA-binding protein CIRP/hnRNP A18 regulates telomerase activity in a temperature-dependent manner.Nucleic Acids Res. 2016 Jan 29;44(2):761-75. doi: 10.1093/nar/gkv1465. Epub 2015 Dec 15. Nucleic Acids Res. 2016. PMID: 26673712 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

