Mechanisms of urokinase plasminogen activator (uPA)-mediated atherosclerosis: role of the uPA receptor and S100A8/A9 proteins
- PMID: 21536666
- PMCID: PMC3121410
- DOI: 10.1074/jbc.M110.202135
Mechanisms of urokinase plasminogen activator (uPA)-mediated atherosclerosis: role of the uPA receptor and S100A8/A9 proteins
Abstract
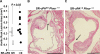
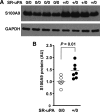
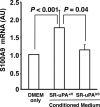
Data from clinical studies, cell culture, and animal models implicate the urokinase plasminogen activator (uPA)/uPA receptor (uPAR)/plasminogen system in the development of atherosclerosis and aneurysms. However, the mechanisms through which uPA/uPAR/plasminogen stimulate these diseases are not yet defined. We used genetically modified, atherosclerosis-prone mice, including mice with macrophage-specific uPA overexpression and mice genetically deficient in uPAR to elucidate mechanisms of uPA/uPAR/plasminogen-accelerated atherosclerosis and aneurysm formation. We found that macrophage-specific uPA overexpression accelerates atherosclerosis and causes aortic root dilation in fat-fed Ldlr(-/-) mice (as we previously reported in Apoe(-/-) mice). Macrophage-expressed uPA accelerates atherosclerosis by stimulation of lesion progression rather than initiation and causes disproportionate lipid accumulation in early lesions. uPA-accelerated atherosclerosis and aortic dilation are largely, if not completely, independent of uPAR. In the absence of uPA overexpression, however, uPAR contributes modestly to both atherosclerosis and aortic dilation. Microarray studies identified S100A8 and S100A9 mRNA as the most highly up-regulated transcripts in uPA-overexpressing macrophages; up-regulation of S100A9 protein in uPA-overexpressing macrophages was confirmed by Western blotting. S100A8/A9, which are atherogenic in mice and are expressed in human atherosclerotic plaques, are also up-regulated in the aortae of mice with uPA-overexpressing macrophages, and macrophage S100A9 mRNA is up-regulated by exposure of wild-type macrophages to medium from uPA-overexpressing macrophages. Macrophage microarray data suggest significant effects of uPA overexpression on cell migration and cell-matrix interactions. Our results confirm in a second animal model that macrophage-expressed uPA stimulates atherosclerosis and aortic dilation. They also reveal uPAR independence of these actions and implicate specific pathways in uPA/Plg-accelerated atherosclerosis and aneurysmal disease.
Figures






Similar articles
-
Reduction of mouse atherosclerosis by urokinase inhibition or with a limited-spectrum matrix metalloproteinase inhibitor.Cardiovasc Res. 2015 Mar 1;105(3):372-82. doi: 10.1093/cvr/cvv007. Epub 2015 Jan 23. Cardiovasc Res. 2015. PMID: 25616415 Free PMC article.
-
Aggregated low-density lipoprotein induce impairment of the cytoskeleton dynamics through urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor in human vascular smooth muscle cell.J Thromb Haemost. 2012 Oct;10(10):2158-67. doi: 10.1111/j.1538-7836.2012.04896.x. J Thromb Haemost. 2012. PMID: 22906080
-
Microglia and the urokinase plasminogen activator receptor/uPA system in innate brain inflammation.Glia. 2009 Dec;57(16):1802-14. doi: 10.1002/glia.20892. Glia. 2009. PMID: 19459212 Free PMC article.
-
uPA/uPAR and SERPINE1 in head and neck cancer: role in tumor resistance, metastasis, prognosis and therapy.Oncotarget. 2016 Aug 30;7(35):57351-57366. doi: 10.18632/oncotarget.10344. Oncotarget. 2016. PMID: 27385000 Free PMC article. Review.
-
Regulation and role of urokinase plasminogen activator in vascular remodelling.Clin Exp Pharmacol Physiol. 1996 Sep;23(9):759-65. doi: 10.1111/j.1440-1681.1996.tb01177.x. Clin Exp Pharmacol Physiol. 1996. PMID: 8911711 Review.
Cited by
-
Plasma Olink Proteomics Reveals Novel Biomarkers for Prediction and Diagnosis in Dilated Cardiomyopathy with Heart Failure.J Proteome Res. 2024 Sep 6;23(9):4139-4150. doi: 10.1021/acs.jproteome.4c00522. Epub 2024 Aug 11. J Proteome Res. 2024. PMID: 39129220
-
Aortopathy in a Mouse Model of Marfan Syndrome Is Not Mediated by Altered Transforming Growth Factor β Signaling.J Am Heart Assoc. 2017 Jan 24;6(1):e004968. doi: 10.1161/JAHA.116.004968. J Am Heart Assoc. 2017. PMID: 28119285 Free PMC article.
-
The uPAR System as a Potential Therapeutic Target in the Diseased Eye.Cells. 2019 Aug 18;8(8):925. doi: 10.3390/cells8080925. Cells. 2019. PMID: 31426601 Free PMC article. Review.
-
S100A8 and S100A9 in cardiovascular biology and disease.Arterioscler Thromb Vasc Biol. 2012 Feb;32(2):223-9. doi: 10.1161/ATVBAHA.111.236927. Epub 2011 Nov 17. Arterioscler Thromb Vasc Biol. 2012. PMID: 22095980 Free PMC article. Review.
-
Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture.Molecules. 2015 Sep 25;20(10):17720-34. doi: 10.3390/molecules201017720. Molecules. 2015. PMID: 26404213 Free PMC article.
References
-
- Virmani R., Burke A. P., Farb A., Kolodgie F. D. (2002) Prog. Cardiovasc. Dis. 44, 349–356 - PubMed
-
- Guo D. C., Papke C. L., He R., Milewicz D. M. (2006) Ann. N.Y. Acad. Sci. 1085, 339–352 - PubMed
-
- Lijnen H. R. (2002) Biochem. Soc. Trans. 30, 163–167 - PubMed
-
- Padró T., Emeis J. J., Steins M., Schmid K. W., Kienast J. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 893–902 - PubMed
-
- Kienast J., Padró T., Steins M., Li C. X., Schmid K. W., Hammel D., Scheld H. H., van de Loo J. C. (1998) Thromb. Haemost. 79, 579–586 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

