Multifaceted intervention by the Hsp90 inhibitor ganetespib (STA-9090) in cancer cells with activated JAK/STAT signaling
- PMID: 21533169
- PMCID: PMC3077378
- DOI: 10.1371/journal.pone.0018552
Multifaceted intervention by the Hsp90 inhibitor ganetespib (STA-9090) in cancer cells with activated JAK/STAT signaling
Abstract
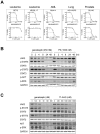
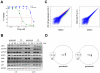
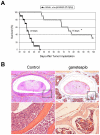
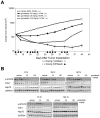
There is accumulating evidence that dysregulated JAK signaling occurs in a wide variety of cancer types. In particular, mutations in JAK2 can result in the constitutive activation of STAT transcription factors and lead to oncogenic growth. JAK kinases are established Hsp90 client proteins and here we show that the novel small molecule Hsp90 inhibitor ganetespib (formerly STA-9090) exhibits potent in vitro and in vivo activity in a range of solid and hematological tumor cells that are dependent on JAK2 activity for growth and survival. Of note, ganetespib treatment results in sustained depletion of JAK2, including the constitutively active JAK2(V617F) mutant, with subsequent loss of STAT activity and reduced STAT-target gene expression. In contrast, treatment with the pan-JAK inhibitor P6 results in only transient effects on these processes. Further differentiating these modes of intervention, RNA and protein expression studies show that ganetespib additionally modulates cell cycle regulatory proteins, while P6 does not. The concomitant impact of ganetespib on both cell growth and cell division signaling translates to potent antitumor efficacy in mouse models of xenografts and disseminated JAK/STAT-driven leukemia. Overall, our findings support Hsp90 inhibition as a novel therapeutic approach for combating diseases dependent on JAK/STAT signaling, with the multimodal action of ganetespib demonstrating advantages over JAK-specific inhibitors.
Conflict of interest statement
Figures


Similar articles
-
Targeting the Janus-activated kinase-2-STAT3 signalling pathway in pancreatic cancer using the HSP90 inhibitor ganetespib.Eur J Cancer. 2016 Jan;52:109-19. doi: 10.1016/j.ejca.2015.10.057. Epub 2015 Dec 9. Eur J Cancer. 2016. PMID: 26682870
-
Overcoming acquired resistance to HSP90 inhibition by targeting JAK-STAT signalling in triple-negative breast cancer.BMC Cancer. 2019 Jan 24;19(1):102. doi: 10.1186/s12885-019-5295-z. BMC Cancer. 2019. PMID: 30678647 Free PMC article.
-
Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy.Mol Cancer Ther. 2012 Feb;11(2):475-84. doi: 10.1158/1535-7163.MCT-11-0755. Epub 2011 Dec 5. Mol Cancer Ther. 2012. PMID: 22144665
-
Recent updates on the development of ganetespib as a Hsp90 inhibitor.Arch Pharm Res. 2012 Nov;35(11):1855-9. doi: 10.1007/s12272-012-1101-z. Arch Pharm Res. 2012. PMID: 23212626 Review.
-
JAK/STAT signaling in hematological malignancies.Oncogene. 2013 May 23;32(21):2601-13. doi: 10.1038/onc.2012.347. Epub 2012 Aug 6. Oncogene. 2013. PMID: 22869151 Review.
Cited by
-
Efficacy of an HSP90 inhibitor, ganetespib, in preclinical thyroid cancer models.Oncotarget. 2017 Jun 20;8(25):41294-41304. doi: 10.18632/oncotarget.17180. Oncotarget. 2017. PMID: 28476040 Free PMC article.
-
Cooperation between oncogenic Ras and wild-type p53 stimulates STAT non-cell autonomously to promote tumor radioresistance.Commun Biol. 2021 Mar 19;4(1):374. doi: 10.1038/s42003-021-01898-5. Commun Biol. 2021. PMID: 33742110 Free PMC article.
-
Gastrointestinal stromal tumors: a review of case reports, diagnosis, treatment, and future directions.ISRN Gastroenterol. 2012;2012:595968. doi: 10.5402/2012/595968. Epub 2012 Apr 12. ISRN Gastroenterol. 2012. PMID: 22577569 Free PMC article.
-
Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer.Cancer Discov. 2013 Apr;3(4):430-43. doi: 10.1158/2159-8290.CD-12-0440. Epub 2013 Mar 26. Cancer Discov. 2013. PMID: 23533265 Free PMC article. Clinical Trial.
-
Role of Ganetespib, an HSP90 Inhibitor, in Cancer Therapy: From Molecular Mechanisms to Clinical Practice.Int J Mol Sci. 2023 Mar 6;24(5):5014. doi: 10.3390/ijms24055014. Int J Mol Sci. 2023. PMID: 36902446 Free PMC article. Review.
References
-
- Rane SG, Reddy EP. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 2002;21:3334–3358. - PubMed
-
- Tefferi A, Gilliland DG. JAK2 in myeloproliferative disorders is not just another kinase. Cell Cycle. 2005;4:1053–1056. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

