A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages
- PMID: 21533069
- PMCID: PMC3080847
- DOI: 10.1371/journal.ppat.1002026
A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages
Abstract
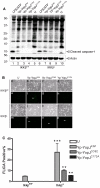
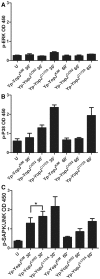
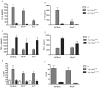
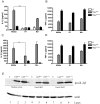
A type III secretion system (T3SS) in pathogenic Yersinia species functions to translocate Yop effectors, which modulate cytokine production and regulate cell death in macrophages. Distinct pathways of T3SS-dependent cell death and caspase-1 activation occur in Yersinia-infected macrophages. One pathway of cell death and caspase-1 activation in macrophages requires the effector YopJ. YopJ is an acetyltransferase that inactivates MAPK kinases and IKKβ to cause TLR4-dependent apoptosis in naïve macrophages. A YopJ isoform in Y. pestis KIM (YopJ(KIM)) has two amino acid substitutions, F177L and K206E, not present in YopJ proteins of Y. pseudotuberculosis and Y. pestis CO92. As compared to other YopJ isoforms, YopJ(KIM) causes increased apoptosis, caspase-1 activation, and secretion of IL-1β in Yersinia-infected macrophages. The molecular basis for increased apoptosis and activation of caspase-1 by YopJ(KIM) in Yersinia-infected macrophages was studied. Site directed mutagenesis showed that the F177L and K206E substitutions in YopJ(KIM) were important for enhanced apoptosis, caspase-1 activation, and IL-1β secretion. As compared to YopJ(CO92), YopJ(KIM) displayed an enhanced capacity to inhibit phosphorylation of IκB-α in macrophages and to bind IKKβ in vitro. YopJ(KIM) also showed a moderately increased ability to inhibit phosphorylation of MAPKs. Increased caspase-1 cleavage and IL-1β secretion occurred in IKKβ-deficient macrophages infected with Y. pestis expressing YopJ(CO92), confirming that the NF-κB pathway can negatively regulate inflammasome activation. K+ efflux, NLRP3 and ASC were important for secretion of IL-1β in response to Y. pestis KIM infection as shown using macrophages lacking inflammasome components or by the addition of exogenous KCl. These data show that caspase-1 is activated in naïve macrophages in response to infection with a pathogen that inhibits IKKβ and MAPK kinases and induces TLR4-dependent apoptosis. This pro-inflammatory form of apoptosis may represent an early innate immune response to highly virulent pathogens such as Y. pestis KIM that have evolved an enhanced ability to inhibit host signaling pathways.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
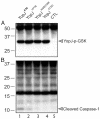
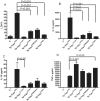
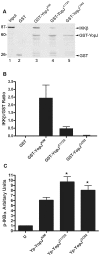
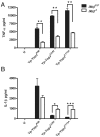
Similar articles
-
YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense.PLoS One. 2012;7(4):e36019. doi: 10.1371/journal.pone.0036019. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563435 Free PMC article.
-
Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ.Infect Immun. 2008 Sep;76(9):3911-23. doi: 10.1128/IAI.01695-07. Epub 2008 Jun 16. Infect Immun. 2008. PMID: 18559430 Free PMC article.
-
Manipulation of Interleukin-1β and Interleukin-18 Production by Yersinia pestis Effectors YopJ and YopM and Redundant Impact on Virulence.J Biol Chem. 2016 May 6;291(19):9894-905. doi: 10.1074/jbc.M115.697698. Epub 2016 Feb 16. J Biol Chem. 2016. PMID: 26884330 Free PMC article.
-
Cross-regulation between the IL-1β/IL-18 processing inflammasome and other inflammatory cytokines.Curr Opin Immunol. 2011 Oct;23(5):591-7. doi: 10.1016/j.coi.2011.07.005. Epub 2011 Aug 10. Curr Opin Immunol. 2011. PMID: 21839623 Free PMC article. Review.
-
Cell intrinsic roles of apoptosis-associated speck-like protein in regulating innate and adaptive immune responses.ScientificWorldJournal. 2011;11:2418-23. doi: 10.1100/2011/429192. Epub 2011 Dec 8. ScientificWorldJournal. 2011. PMID: 22194672 Free PMC article. Review.
Cited by
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Sensing and reacting to microbes through the inflammasomes.Nat Immunol. 2012 Mar 19;13(4):325-32. doi: 10.1038/ni.2231. Nat Immunol. 2012. PMID: 22430785 Free PMC article. Review.
-
Integrin-mediated first signal for inflammasome activation in intestinal epithelial cells.J Immunol. 2014 Aug 1;193(3):1373-82. doi: 10.4049/jimmunol.1400145. Epub 2014 Jun 25. J Immunol. 2014. PMID: 24965773 Free PMC article.
-
Bacterial programming of host responses: coordination between type I interferon and cell death.Front Microbiol. 2014 Oct 28;5:545. doi: 10.3389/fmicb.2014.00545. eCollection 2014. Front Microbiol. 2014. PMID: 25389418 Free PMC article. Review.
-
The Yersinia pestis Effector YopM Inhibits Pyrin Inflammasome Activation.PLoS Pathog. 2016 Dec 2;12(12):e1006035. doi: 10.1371/journal.ppat.1006035. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27911947 Free PMC article.
References
-
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. - PubMed
-
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. - PubMed
-
- Navarre WW, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2000;2:265–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

