Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells
- PMID: 21526224
- PMCID: PMC3079587
- DOI: 10.1371/journal.pbio.1001048
Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells
Abstract
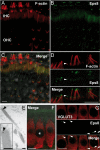
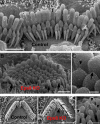
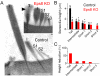
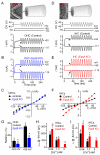
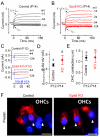
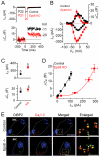
Hair cells of the mammalian cochlea are specialized for the dynamic coding of sound stimuli. The transduction of sound waves into electrical signals depends upon mechanosensitive hair bundles that project from the cell's apical surface. Each stereocilium within a hair bundle is composed of uniformly polarized and tightly packed actin filaments. Several stereociliary proteins have been shown to be associated with hair bundle development and function and are known to cause deafness in mice and humans when mutated. The growth of the stereociliar actin core is dynamically regulated at the actin filament barbed ends in the stereociliary tip. We show that Eps8, a protein with actin binding, bundling, and barbed-end capping activities in other systems, is a novel component of the hair bundle. Eps8 is localized predominantly at the tip of the stereocilia and is essential for their normal elongation and function. Moreover, we have found that Eps8 knockout mice are profoundly deaf and that IHCs, but not OHCs, fail to mature into fully functional sensory receptors. We propose that Eps8 directly regulates stereocilia growth in hair cells and also plays a crucial role in the physiological maturation of mammalian cochlear IHCs. Together, our results indicate that Eps8 is critical in coordinating the development and functionality of mammalian auditory hair cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The actin-binding proteins eps8 and gelsolin have complementary roles in regulating the growth and stability of mechanosensory hair bundles of mammalian cochlear outer hair cells.PLoS One. 2014 Jan 27;9(1):e87331. doi: 10.1371/journal.pone.0087331. eCollection 2014. PLoS One. 2014. PMID: 24475274 Free PMC article.
-
Loss of Baiap2l2 destabilizes the transducing stereocilia of cochlear hair cells and leads to deafness.J Physiol. 2021 Feb;599(4):1173-1198. doi: 10.1113/JP280670. Epub 2020 Nov 26. J Physiol. 2021. PMID: 33151556 Free PMC article.
-
Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2.Proc Natl Acad Sci U S A. 2013 Aug 20;110(34):13898-903. doi: 10.1073/pnas.1304644110. Epub 2013 Aug 5. Proc Natl Acad Sci U S A. 2013. PMID: 23918390 Free PMC article.
-
Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle.Int J Mol Sci. 2020 Jan 3;21(1):324. doi: 10.3390/ijms21010324. Int J Mol Sci. 2020. PMID: 31947734 Free PMC article. Review.
-
The actin cytoskeleton in hair bundle development and hearing loss.Hear Res. 2023 Sep 1;436:108817. doi: 10.1016/j.heares.2023.108817. Epub 2023 May 26. Hear Res. 2023. PMID: 37300948 Free PMC article. Review.
Cited by
-
Eps8 controls dendritic spine density and synaptic plasticity through its actin-capping activity.EMBO J. 2013 Jun 12;32(12):1730-44. doi: 10.1038/emboj.2013.107. Epub 2013 May 17. EMBO J. 2013. PMID: 23685357 Free PMC article.
-
The acquisition of mechano-electrical transducer current adaptation in auditory hair cells requires myosin VI.J Physiol. 2016 Jul 1;594(13):3667-81. doi: 10.1113/JP272220. Epub 2016 May 27. J Physiol. 2016. PMID: 27111754 Free PMC article.
-
EPS8 variant causes deafness, autosomal recessive 102 (DFNB102) and literature review.Hum Genome Var. 2023 Jan 13;10(1):1. doi: 10.1038/s41439-023-00229-w. Hum Genome Var. 2023. PMID: 36635257 Free PMC article.
-
GPSM2-GNAI Specifies the Tallest Stereocilia and Defines Hair Bundle Row Identity.Curr Biol. 2019 Mar 18;29(6):921-934.e4. doi: 10.1016/j.cub.2019.01.051. Epub 2019 Feb 28. Curr Biol. 2019. PMID: 30827920 Free PMC article.
-
Coupling of the mechanotransduction machinery and F-actin polymerization in the cochlear hair bundles.Bioarchitecture. 2011 Jul;1(4):169-174. doi: 10.4161/bioa.1.4.17532. Epub 2011 Jul 1. Bioarchitecture. 2011. PMID: 22069509 Free PMC article.
References
-
- Fettiplace R, Hackney C. M. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7:19–29. - PubMed
-
- Tinley L. G, Tinley M. S, DeRosier D. Actin filaments, stereocilia, and hair cells: how cells count and measure. Ann Rev Cell Biol. 1992;8:257–274. - PubMed
-
- Belyantseva I. A, Labay V, Boger E. T, Griffith A. J, Friedman T. B. Stereocilia: the long and the short of it. Trends Mol Med. 2003;9:458–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

