Biochemical, proteomic, structural, and thermodynamic characterizations of integrin-linked kinase (ILK): cross-validation of the pseudokinase
- PMID: 21524996
- PMCID: PMC3122243
- DOI: 10.1074/jbc.M111.240093
Biochemical, proteomic, structural, and thermodynamic characterizations of integrin-linked kinase (ILK): cross-validation of the pseudokinase
Abstract
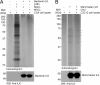
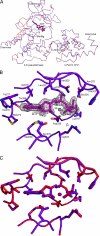
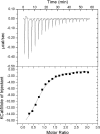
Integrin-linked kinase (ILK) is one of the few evolutionarily conserved focal adhesion proteins involved in diverse cell adhesion-dependent physiological and pathological responses. Despite more than a decade of studies and extensive literature, the kinase function of ILK is controversial. ILK contains a highly degraded kinase active site but it has been argued that ILK may be an unusual manganese (Mn)-dependent serine-threonine kinase that targets specific substrates such as glycogen synthase kinase-3β (GSK-3β). In this study, we have tackled this issue by a systematic bottom-up biochemical, proteomic, structural, and thermodynamic analysis of ILK. We show that recombinant ILK from either bacteria or mammalian cells exhibits no kinase activity on GSK-3β in the presence of either Mn(2+) or the conventional kinase co-factor Mg(2+). A comprehensive and unbiased whole cell-based kinase assay using entire mammalian CG-4 and C2C12 cell lysate did not identify any specific ILK substrates. High resolution crystallographic structure analysis further confirmed that the Mn-bound ILK adopts the same pseudo active site conformation as that of the Mg-bound ILK. More importantly, thermodynamic analysis revealed that the K220M mutation, previously thought to inactivate ILK by disrupting ATP binding, significantly impairs the structural integrity and stability of ILK, which provides a new basis for understanding how this mutation caused renal agenesis, a failure of fetal kidney development. Collectively, our data provide strong evidence that ILK lacks intrinsic kinase function. It is a bona fide pseudokinase that likely evolved from an ancestral catalytic counterpart to act as a distinct scaffold to mediate protein-protein interactions during focal adhesion assembly and many other cellular events.
Figures


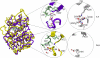
Similar articles
-
Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3beta) phosphorylation.PLoS One. 2010 Aug 23;5(8):e12356. doi: 10.1371/journal.pone.0012356. PLoS One. 2010. PMID: 20827300 Free PMC article.
-
Inactivation of integrin-linked kinase induces aberrant tau phosphorylation via sustained activation of glycogen synthase kinase 3beta in N1E-115 neuroblastoma cells.J Biol Chem. 2003 Jul 18;278(29):26970-5. doi: 10.1074/jbc.M304113200. Epub 2003 Apr 24. J Biol Chem. 2003. PMID: 12714590
-
Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro.Br J Cancer. 2003 May 6;88(9):1470-9. doi: 10.1038/sj.bjc.6600912. Br J Cancer. 2003. PMID: 12778079 Free PMC article.
-
Integrin-linked kinase: not so 'pseudo' after all.Oncogene. 2011 Oct 27;30(43):4375-85. doi: 10.1038/onc.2011.177. Epub 2011 May 23. Oncogene. 2011. PMID: 21602880 Review.
-
The role of integrin-linked kinase (ILK) in cancer progression.Cancer Metastasis Rev. 2003 Dec;22(4):375-84. doi: 10.1023/a:1023777013659. Cancer Metastasis Rev. 2003. PMID: 12884912 Review.
Cited by
-
A robust methodology to subclassify pseudokinases based on their nucleotide-binding properties.Biochem J. 2014 Jan 15;457(2):323-34. doi: 10.1042/BJ20131174. Biochem J. 2014. PMID: 24107129 Free PMC article.
-
Integrin Activation: Implications for Axon Regeneration.Cells. 2018 Mar 10;7(3):20. doi: 10.3390/cells7030020. Cells. 2018. PMID: 29534450 Free PMC article. Review.
-
Insights into the Structure, Function, and Ion-Mediated Signaling Pathways Transduced by Plant Integrin-Linked Kinases.Front Plant Sci. 2017 Apr 3;8:376. doi: 10.3389/fpls.2017.00376. eCollection 2017. Front Plant Sci. 2017. PMID: 28421082 Free PMC article.
-
PINCH1 Promotes Fibroblast Migration in Extracellular Matrices and Influences Their Mechanophenotype.Front Cell Dev Biol. 2022 May 16;10:869563. doi: 10.3389/fcell.2022.869563. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35652097 Free PMC article.
-
Vascular mechanotransduction.Physiol Rev. 2023 Apr 1;103(2):1247-1421. doi: 10.1152/physrev.00053.2021. Epub 2023 Jan 5. Physiol Rev. 2023. PMID: 36603156 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

