Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine
- PMID: 21513749
- PMCID: PMC3716467
- DOI: 10.1016/j.jconrel.2011.04.007
Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine
Abstract
The development of injectable and biocompatible vehicles for delivery, retention, growth, and differentiation of stem cells is of paramount importance for regenerative medicine. For cell therapy and the development of clinical combination products, we created a hyaluronan (HA)-based synthetic extracellular matrix (sECM) that provides highly reproducible, manufacturable, approvable, and affordable biomaterials. The composition of the sECM can be customized for use with progenitor and mature cell populations obtained from skin, fat, liver, heart, muscle, bone, cartilage, nerves, and other tissues. This overview describes the design criteria for "living" HA derivatives, and the many uses of this in situ crosslinkable HA-based sECM hydrogel for three-dimensional (3-D) culture of cells in vitro and translational use in vivo. Recent advances allow rapid expansion and recovery of cells in 3-D, and the bioprinting of engineered tissue constructs. The uses of HA-derived sECMs for cell and molecule delivery in vivo will be reviewed, including applications in cancer biology and tumor imaging.
Copyright © 2011. Published by Elsevier B.V.
Figures


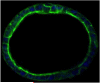

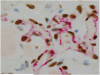
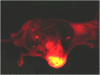
Similar articles
-
Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery.Acc Chem Res. 2008 Jan;41(1):139-48. doi: 10.1021/ar7000827. Epub 2007 Jul 27. Acc Chem Res. 2008. PMID: 17655274 Review.
-
Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering.J Biomed Mater Res A. 2006 Dec 15;79(4):902-12. doi: 10.1002/jbm.a.30831. J Biomed Mater Res A. 2006. PMID: 16941590
-
Modular extracellular matrices: solutions for the puzzle.Methods. 2008 May;45(1):93-8. doi: 10.1016/j.ymeth.2008.01.010. Methods. 2008. PMID: 18442709 Free PMC article.
-
Engineering a clinically-useful matrix for cell therapy.Organogenesis. 2008 Jan;4(1):42-7. doi: 10.4161/org.6152. Organogenesis. 2008. PMID: 19279714 Free PMC article.
-
Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine.Int J Biol Macromol. 2016 May;86:917-28. doi: 10.1016/j.ijbiomac.2016.02.032. Epub 2016 Feb 15. Int J Biol Macromol. 2016. PMID: 26893053 Review.
Cited by
-
A high molecular weight hyaluronic acid biphasic dispersion as potential therapeutics for interstitial cystitis.J Biomed Mater Res B Appl Biomater. 2021 Jun;109(6):864-876. doi: 10.1002/jbm.b.34751. Epub 2020 Oct 26. J Biomed Mater Res B Appl Biomater. 2021. PMID: 33103826 Free PMC article.
-
Lyophilized Progenitor Tenocyte Extracts: Sterilizable Cytotherapeutic Derivatives with Antioxidant Properties and Hyaluronan Hydrogel Functionalization Effects.Antioxidants (Basel). 2023 Jan 10;12(1):163. doi: 10.3390/antiox12010163. Antioxidants (Basel). 2023. PMID: 36671025 Free PMC article.
-
Advances in Hyaluronic Acid for Biomedical Applications.Front Bioeng Biotechnol. 2022 Jul 4;10:910290. doi: 10.3389/fbioe.2022.910290. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35860333 Free PMC article. Review.
-
Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells.Acta Biomater. 2014 May;10(5):1836-46. doi: 10.1016/j.actbio.2013.12.005. Epub 2013 Dec 12. Acta Biomater. 2014. PMID: 24334142 Free PMC article.
-
Biomimetic Material-Assisted Delivery of Human Embryonic Stem Cell Derivatives for Enhanced In Vivo Survival and Engraftment.ACS Biomater Sci Eng. 2015 Jan 12;1(1):7-12. doi: 10.1021/ab500021a. ACS Biomater Sci Eng. 2015. PMID: 26280019 Free PMC article.
References
-
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. - PubMed
-
- Kuo JW. Practical aspects of hyaluronan based medical products. CRC/Taylor & Francis; Boca Raton: 2006.
-
- Allison DD, Grande-Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–2140. - PubMed
-
- Condie RC, Prestwich GD. Engineering Clinically Useful Hyaluronan Matrices. In: Vernon B, editor. Injectable Biomaterials: Science and Application. Woodhead Publishing; London: 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

