The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota
- PMID: 21512004
- PMCID: PMC3164325
- DOI: 10.1126/science.1206095
The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota
Abstract
Mucosal surfaces constantly encounter microbes. Toll-like receptors (TLRs) mediate recognition of microbial patterns to eliminate pathogens. By contrast, we demonstrate that the prominent gut commensal Bacteroides fragilis activates the TLR pathway to establish host-microbial symbiosis. TLR2 on CD4(+) T cells is required for B. fragilis colonization of a unique mucosal niche in mice during homeostasis. A symbiosis factor (PSA, polysaccharide A) of B. fragilis signals through TLR2 directly on Foxp3(+) regulatory T cells to promote immunologic tolerance. B. fragilis lacking PSA is unable to restrain T helper 17 cell responses and is defective in niche-specific mucosal colonization. Therefore, commensal bacteria exploit the TLR pathway to actively suppress immunity. We propose that the immune system can discriminate between pathogens and the microbiota through recognition of symbiotic bacterial molecules in a process that engenders commensal colonization.
Figures

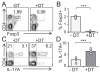
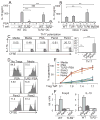
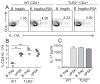
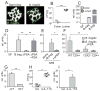
Comment in
-
Commensal microbiota determine intestinal iTreg.Am J Transplant. 2012 Aug;12(8):1967. doi: 10.1111/j.1600-6143.2012.04217.x. Am J Transplant. 2012. PMID: 22845904 No abstract available.
Similar articles
-
Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota.Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12204-9. doi: 10.1073/pnas.0909122107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566854 Free PMC article.
-
Gut microbiota utilize immunoglobulin A for mucosal colonization.Science. 2018 May 18;360(6390):795-800. doi: 10.1126/science.aaq0926. Epub 2018 May 3. Science. 2018. PMID: 29724905 Free PMC article.
-
Bacterial colonization factors control specificity and stability of the gut microbiota.Nature. 2013 Sep 19;501(7467):426-9. doi: 10.1038/nature12447. Epub 2013 Aug 18. Nature. 2013. PMID: 23955152 Free PMC article.
-
Beneficial effects of Bacteroides fragilis polysaccharides on the immune system.Front Biosci (Landmark Ed). 2010 Jan 1;15(1):25-34. doi: 10.2741/3603. Front Biosci (Landmark Ed). 2010. PMID: 20036803 Free PMC article. Review.
-
Finding a needle in a haystack: Bacteroides fragilis polysaccharide A as the archetypical symbiosis factor.Ann N Y Acad Sci. 2018 Apr;1417(1):116-129. doi: 10.1111/nyas.13660. Epub 2018 Mar 12. Ann N Y Acad Sci. 2018. PMID: 29528123 Review.
Cited by
-
Macrophage cytokine responses to commensal Gram-positive Lactobacillus salivarius strains are TLR2-independent and Myd88-dependent.Sci Rep. 2021 Mar 15;11(1):5896. doi: 10.1038/s41598-021-85347-7. Sci Rep. 2021. PMID: 33723368 Free PMC article.
-
The gut microbiome in multiple sclerosis.Curr Treat Options Neurol. 2015 Apr;17(4):344. doi: 10.1007/s11940-015-0344-7. Curr Treat Options Neurol. 2015. PMID: 25843302
-
Beyond pattern recognition: five immune checkpoints for scaling the microbial threat.Nat Rev Immunol. 2012 Feb 24;12(3):215-25. doi: 10.1038/nri3167. Nat Rev Immunol. 2012. PMID: 22362354
-
Gut microbiota shed new light on the management of immune-related adverse events.Thorac Cancer. 2022 Oct;13(19):2681-2691. doi: 10.1111/1759-7714.14626. Epub 2022 Aug 31. Thorac Cancer. 2022. PMID: 36043345 Free PMC article. Review.
-
Upper gastrointestinal microbiota and digestive diseases.World J Gastroenterol. 2013 Mar 14;19(10):1541-50. doi: 10.3748/wjg.v19.i10.1541. World J Gastroenterol. 2013. PMID: 23539678 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI088626-02/AI/NIAID NIH HHS/United States
- R01 DK078938-02/DK/NIDDK NIH HHS/United States
- R01 AI085090-02/AI/NIAID NIH HHS/United States
- R21 AI080002/AI/NIAID NIH HHS/United States
- R01 AI085090/AI/NIAID NIH HHS/United States
- R01 DK078938-04/DK/NIDDK NIH HHS/United States
- R21 AI080002-02/AI/NIAID NIH HHS/United States
- DK 083633/DK/NIDDK NIH HHS/United States
- R21 DK083633/DK/NIDDK NIH HHS/United States
- AI 088626/AI/NIAID NIH HHS/United States
- R21 DK083633-02/DK/NIDDK NIH HHS/United States
- R01 DK078938-03/DK/NIDDK NIH HHS/United States
- R01 AI085090-03/AI/NIAID NIH HHS/United States
- R01 DK078938-01A2/DK/NIDDK NIH HHS/United States
- R01 AI085090-01S1/AI/NIAID NIH HHS/United States
- DK 078938/DK/NIDDK NIH HHS/United States
- R21 DK083633-01A1/DK/NIDDK NIH HHS/United States
- R01 AI085090-01/AI/NIAID NIH HHS/United States
- R21 AI088626/AI/NIAID NIH HHS/United States
- AI 080002/AI/NIAID NIH HHS/United States
- R21 AI080002-01/AI/NIAID NIH HHS/United States
- R21 AI088626-01/AI/NIAID NIH HHS/United States
- R01 DK078938/DK/NIDDK NIH HHS/United States
- R56 DK078938/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

