CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease
- PMID: 21508244
- PMCID: PMC6632958
- DOI: 10.1523/JNEUROSCI.0299-11.2011
CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease
Abstract
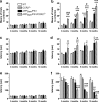
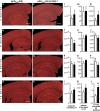
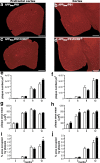
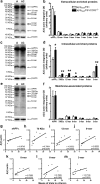
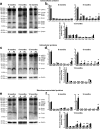
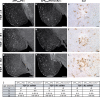
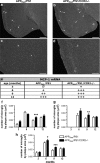
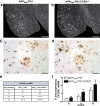
Circulating monocytoid cells have the ability to infiltrate nervous tissue, differentiate into microglia, and clear amyloid-β (Aβ) from the brain of mouse models of Alzheimer's disease. Interaction between the chemokine CCL2 and its CC chemokine receptor 2 (CCR2) plays a critical role in the recruitment of inflammatory monocytes into the injured/diseased brain. Here, we show that CCR2 deficiency aggravates mnesic deficits and amyloid pathology in transgenic mice expressing the chimeric mouse/human β-amyloid precursor protein and presenilin 1 (APP(Swe)/PS1). Indeed, memory impairment was accelerated and enhanced in APP(Swe)/PS1/CCR2(-/-) mice. Apparition of cognitive decline occurred earlier (i.e., at 3 months of age before plaque formation) and correlated with intracellular accumulation of soluble oligomeric forms of Aβ. Memory deficits worsened with age and were aggravated in APP(Swe)/PS1/CCR2(-/-) mice compared with their respective control groups. Soluble Aβ assemblies increased significantly in APP(Swe)/PS1 mice in a context of CCR2 deficiency, whereas the plaque load remained relatively similar in the brain of aging APP(Swe)/PS1 and APP(Swe)/PS1/CCR2(-/-) mice. However, CCR2 deficiency stimulated the expression of TGF-β1, TGF-β receptors, and CX(3)CR1 transcripts in plaque-associated microglia, a pattern that is characteristic of an antiinflammatory subset of myeloid cells. A decreased expression of CCR2 could play a potential role in the etiology of Alzheimer's disease, a neurodegenerative pathology that could be treated by a genetic upregulation of the transgene in monocytoid cells.
Figures
Similar articles
-
Hematopoietic CC-chemokine receptor 2 (CCR2) competent cells are protective for the cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease.Mol Med. 2012 Mar 30;18(1):297-313. doi: 10.2119/molmed.2011.00306. Mol Med. 2012. PMID: 22160221 Free PMC article.
-
Interleukin-1β mediated amyloid plaque clearance is independent of CCR2 signaling in the APP/PS1 mouse model of Alzheimer's disease.Neurobiol Dis. 2014 Sep;69:124-33. doi: 10.1016/j.nbd.2014.05.018. Epub 2014 May 27. Neurobiol Dis. 2014. PMID: 24874542 Free PMC article.
-
Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer's disease.J Neurosci. 2008 May 28;28(22):5784-93. doi: 10.1523/JNEUROSCI.1146-08.2008. J Neurosci. 2008. PMID: 18509040 Free PMC article.
-
Decoding the role of the CCL2/CCR2 axis in Alzheimer's disease and innovating therapeutic approaches: Keeping All options open.Int Immunopharmacol. 2024 Jun 30;135:112328. doi: 10.1016/j.intimp.2024.112328. Epub 2024 May 25. Int Immunopharmacol. 2024. PMID: 38796962 Review.
-
When neurogenesis encounters aging and disease.Trends Neurosci. 2010 Dec;33(12):569-79. doi: 10.1016/j.tins.2010.09.003. Epub 2010 Oct 18. Trends Neurosci. 2010. PMID: 20961627 Free PMC article. Review.
Cited by
-
Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques.Nat Commun. 2015 Jan 29;6:6176. doi: 10.1038/ncomms7176. Nat Commun. 2015. PMID: 25630253 Free PMC article.
-
Innate immunity in Alzheimer's disease: a complex affair.CNS Neurol Disord Drug Targets. 2013 Aug;12(5):593-607. doi: 10.2174/1871527311312050008. CNS Neurol Disord Drug Targets. 2013. PMID: 23574177 Free PMC article. Review.
-
The blood-brain barrier in Alzheimer's disease.Neurobiol Dis. 2017 Nov;107:41-56. doi: 10.1016/j.nbd.2016.07.007. Epub 2016 Jul 15. Neurobiol Dis. 2017. PMID: 27425887 Free PMC article. Review.
-
Nuclear receptors as therapeutic targets for Alzheimer's disease.Expert Opin Ther Targets. 2011 Sep;15(9):1085-97. doi: 10.1517/14728222.2011.594043. Epub 2011 Jul 1. Expert Opin Ther Targets. 2011. PMID: 21718217 Free PMC article. Review.
-
Arginine deprivation and immune suppression in a mouse model of Alzheimer's disease.J Neurosci. 2015 Apr 15;35(15):5969-82. doi: 10.1523/JNEUROSCI.4668-14.2015. J Neurosci. 2015. PMID: 25878270 Free PMC article.
References
-
- Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. - PMC - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S. Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer's disease. Brain. 2009;132:1078–1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
