Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells
- PMID: 21508222
- PMCID: PMC3091622
- DOI: 10.1523/JNEUROSCI.4441-10.2011
Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells
Abstract
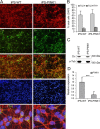
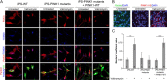
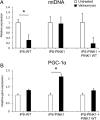
Genetic Parkinson disease (PD) has been associated with mutations in PINK1, a gene encoding a mitochondrial kinase implicated in the regulation of mitochondrial degradation. While the studies so far examined PINK1 function in non-neuronal systems or through PINK1 knockdown approaches, there is an imperative to examine the role of endogenous PINK1 in appropriate human-derived and biologically relevant cell models. Here we report the generation of induced pluripotent stem (iPS) cells from skin fibroblasts taken from three PD patients with nonsense (c.1366C>T; p.Q456X) or missense (c.509T>G; p.V170G) mutations in the PINK1 gene. These cells were differentiated into dopaminergic neurons that upon mitochondrial depolarization showed impaired recruitment of lentivirally expressed Parkin to mitochondria, increased mitochondrial copy number, and upregulation of PGC-1α, an important regulator of mitochondrial biogenesis. Importantly, these alterations were corrected by lentiviral expression of wild-type PINK1 in mutant iPS cell-derived PINK1 neurons. In conclusion, our studies suggest that fibroblasts from genetic PD can be reprogrammed and differentiated into neurons. These neurons exhibit distinct phenotypes that should be amenable to further mechanistic studies in this relevant biological context.
Figures

Similar articles
-
Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)-dependent ubiquitination of endogenous Parkin attenuates mitophagy: study in human primary fibroblasts and induced pluripotent stem cell-derived neurons.J Biol Chem. 2013 Jan 25;288(4):2223-37. doi: 10.1074/jbc.M112.391680. Epub 2012 Dec 4. J Biol Chem. 2013. PMID: 23212910 Free PMC article.
-
Effect of endogenous mutant and wild-type PINK1 on Parkin in fibroblasts from Parkinson disease patients.Hum Mol Genet. 2010 Aug 15;19(16):3124-37. doi: 10.1093/hmg/ddq215. Epub 2010 May 27. Hum Mol Genet. 2010. PMID: 20508036
-
The principal PINK1 and Parkin cellular events triggered in response to dissipation of mitochondrial membrane potential occur in primary neurons.Genes Cells. 2013 Aug;18(8):672-81. doi: 10.1111/gtc.12066. Epub 2013 Jun 10. Genes Cells. 2013. PMID: 23751051 Free PMC article.
-
Mechanisms of neurodegeneration in Parkinson's disease: keep neurons in the PINK1.Mech Ageing Dev. 2020 Jul;189:111277. doi: 10.1016/j.mad.2020.111277. Epub 2020 Jun 3. Mech Ageing Dev. 2020. PMID: 32504621 Review.
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
Cited by
-
Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences.EMBO J. 2012 Jun 26;31(14):3038-62. doi: 10.1038/emboj.2012.170. EMBO J. 2012. PMID: 22735187 Free PMC article. Review.
-
Generation and applications of human pluripotent stem cells induced into neural lineages and neural tissues.Front Physiol. 2012 Mar 19;3:47. doi: 10.3389/fphys.2012.00047. eCollection 2012. Front Physiol. 2012. PMID: 22457650 Free PMC article.
-
Dysregulation of mitochondrial and proteolysosomal genes in Parkinson's disease myeloid cells.Nat Aging. 2021 Sep;1(9):850-863. doi: 10.1038/s43587-021-00110-x. Epub 2021 Sep 14. Nat Aging. 2021. PMID: 35005630 Free PMC article.
-
Nanopore Single-Molecule Sequencing for Mitochondrial DNA Methylation Analysis: Investigating Parkin-Associated Parkinsonism as a Proof of Concept.Front Aging Neurosci. 2021 Sep 28;13:713084. doi: 10.3389/fnagi.2021.713084. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34650424 Free PMC article.
-
A monolayer hiPSC culture system for autophagy/mitophagy studies in human dopaminergic neurons.Autophagy. 2021 Apr;17(4):855-871. doi: 10.1080/15548627.2020.1739441. Epub 2020 Apr 14. Autophagy. 2021. PMID: 32286126 Free PMC article.
References
-
- Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem. 2010;285:21590–21599. - PMC - PubMed
-
- Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. - PubMed
-
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. - PubMed
-
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

