Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence
- PMID: 21508015
- PMCID: PMC3177613
- DOI: 10.1098/rsif.2011.0062
Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence
Abstract
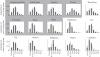
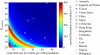
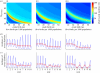
Rotavirus is a major cause of mortality in developing countries, and yet the dynamics of rotavirus in such settings are poorly understood. Rotavirus is typically less seasonal in the tropics, although recent observational studies have challenged the universality of this pattern. While numerous studies have examined the association between environmental factors and rotavirus incidence, here we explore the role of intrinsic factors. By fitting a mathematical model of rotavirus transmission dynamics to published age distributions of cases from 15 countries, we obtain estimates of local transmission rates. Model-predicted patterns of seasonal incidence based solely on differences in birth rates and transmission rates are significantly correlated with those observed (Spearman's ρ = 0.65, p < 0.05). We then examine seasonal patterns of rotavirus predicted across a range of different birth rates and transmission rates and explore how vaccination may impact these patterns. Our results suggest that the relative lack of rotavirus seasonality observed in many tropical countries may be due to the high birth rates and transmission rates typical of developing countries rather than being driven primarily by environmental conditions. While vaccination is expected to decrease the overall burden of disease, it may increase the degree of seasonal variation in the incidence of rotavirus in some settings.
Figures

Similar articles
-
Modelling the seasonality of rotavirus disease and the impact of vaccination in England and Wales.Vaccine. 2010 Apr 19;28(18):3118-26. doi: 10.1016/j.vaccine.2010.02.060. Epub 2010 Mar 1. Vaccine. 2010. PMID: 20197142
-
The influence of demographic and meteorological factors on temporal patterns of rotavirus infection in Dhaka, Bangladesh.Proc Biol Sci. 2022 Jun 8;289(1976):20212727. doi: 10.1098/rspb.2021.2727. Epub 2022 Jun 8. Proc Biol Sci. 2022. PMID: 35673869 Free PMC article.
-
Global seasonality of rotavirus disease.Pediatr Infect Dis J. 2013 Apr;32(4):e134-47. doi: 10.1097/INF.0b013e31827d3b68. Pediatr Infect Dis J. 2013. PMID: 23190782 Free PMC article.
-
Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis.Int J Epidemiol. 2009 Dec;38(6):1487-96. doi: 10.1093/ije/dyn260. Epub 2008 Dec 4. Int J Epidemiol. 2009. PMID: 19056806 Free PMC article. Review.
-
Rotavirus infections and vaccines: burden of illness and potential impact of vaccination.Paediatr Drugs. 2010 Aug 1;12(4):235-56. doi: 10.2165/11537200-000000000-00000. Paediatr Drugs. 2010. PMID: 20593908 Review.
Cited by
-
Evaluating strategies to improve rotavirus vaccine impact during the second year of life in Malawi.Sci Transl Med. 2019 Aug 14;11(505):eaav6419. doi: 10.1126/scitranslmed.aav6419. Sci Transl Med. 2019. PMID: 31413144 Free PMC article.
-
First detection of a reassortant G3P[8] rotavirus A strain in Italy: a case report in an 8-year-old child.Virol J. 2019 May 15;16(1):64. doi: 10.1186/s12985-019-1173-1. Virol J. 2019. PMID: 31092258 Free PMC article.
-
Norovirus transmission dynamics: a modelling review.Epidemiol Infect. 2018 Jan;146(2):147-158. doi: 10.1017/S0950268817002692. Epub 2017 Dec 22. Epidemiol Infect. 2018. PMID: 29268812 Free PMC article. Review.
-
Enteric Viruses in Surface Waters from Argentina: Molecular and Viable-Virus Detection.Appl Environ Microbiol. 2018 Feb 14;84(5):e02327-17. doi: 10.1128/AEM.02327-17. Print 2018 Mar 1. Appl Environ Microbiol. 2018. PMID: 29269500 Free PMC article.
-
Unbiased whole-genome deep sequencing of human and porcine stool samples reveals circulation of multiple groups of rotaviruses and a putative zoonotic infection.Virus Evol. 2016 Oct 3;2(2):vew027. doi: 10.1093/ve/vew027. eCollection 2016 Jul. Virus Evol. 2016. PMID: 28748110 Free PMC article.
References
-
- Glass R. I., Parashar U. D., Bresee J. S., Turcios R., Fischer T. K., Widdowson M. A., Jiang B., Gentsch J. R. 2006. Rotavirus vaccines: current prospects and future challenges. Lancet 368, 323–33210.1016/S0140-6736(06)68815-6 (doi:10.1016/S0140-6736(06)68815-6) - DOI - DOI - PubMed
-
- Tate J. E., et al. 2010. Global impact of rotavirus vaccines. Expert. Rev. Vaccines 9, 395–40710.1586/erv.10.17 (doi:10.1586/erv.10.17) - DOI - DOI - PubMed
-
- Armah G. E., et al. 2010. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 376, 606–61410.1016/s0140-6736(10)60889-6 (doi:10.1016/s0140-6736(10)60889-6). - DOI - DOI - PubMed
-
- Linhares A. C., et al. 2008. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 371, 1181–118910.1016/S0140-6736(08)60524-3 (doi:10.1016/S0140-6736(08)60524-3) - DOI - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

