Casein kinase 2 regulates the mRNA-destabilizing activity of tristetraprolin
- PMID: 21507959
- PMCID: PMC3122216
- DOI: 10.1074/jbc.M110.201137
Casein kinase 2 regulates the mRNA-destabilizing activity of tristetraprolin
Abstract
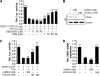
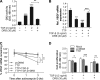
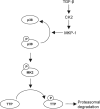
Tristetraprolin (TTP) is an AU-rich element-binding protein that regulates mRNA stability. We previously showed that TTP acts as a negative regulator of VEGF gene expression in colon cancer cells. The p38 MAPK pathway is known to suppress the TTP activity. However, until now the signaling pathway to enhance TTP function is not well known. Here, we show that casein kinase 2 (CK2) enhances the TTP function in the regulation of the VEGF expression in colon cancer cells. CK2 increased TTP protein levels and enhanced VEGF mRNA decaying activity of TTP. TTP was not a direct target of CK2. Instead, CK2 increased the phosphorylation of MKP-1, which led to a decrease in the phosphorylation of p38 MAPK. Inhibition of MKP-1 by siRNA attenuated the increase in TTP function and the decrease of p38 phosphorylation induced by CK2α overexpression. TGF-β1 increased the expressions of CK2 and TTP and the TTP function. The siRNA against CK2α or TTP reversed TGF-β1-induced increases in the expression of CK2 and TTP and the TTP function. Our data suggest that CK2 enhances the protein level and activity of TTP via the modulation of the MKP-1-p38 MAPK signaling pathway and that TGF-β1 enhances the activity of CK2.
Figures
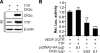
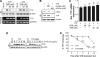

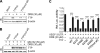

Similar articles
-
Regulation of tristetraprolin expression by mitogen-activated protein kinase phosphatase-1.APMIS. 2012 Dec;120(12):988-99. doi: 10.1111/j.1600-0463.2012.02927.x. Epub 2012 Jun 24. APMIS. 2012. PMID: 23030431
-
Temporal regulation of cytokine mRNA expression by tristetraprolin: dynamic control by p38 MAPK and MKP-1.Am J Physiol Lung Cell Mol Physiol. 2015 May 1;308(9):L973-80. doi: 10.1152/ajplung.00219.2014. Epub 2015 Feb 27. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25724669
-
Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer.Int J Cancer. 2010 Apr 15;126(8):1817-1827. doi: 10.1002/ijc.24847. Int J Cancer. 2010. PMID: 19697322
-
MAPK p38 regulates inflammatory gene expression via tristetraprolin: Doing good by stealth.Int J Biochem Cell Biol. 2018 Jan;94:6-9. doi: 10.1016/j.biocel.2017.11.003. Epub 2017 Nov 8. Int J Biochem Cell Biol. 2018. PMID: 29128684 Free PMC article. Review.
-
Control of mRNA decay by phosphorylation of tristetraprolin.Biochem Soc Trans. 2008 Jun;36(Pt 3):491-6. doi: 10.1042/BST0360491. Biochem Soc Trans. 2008. PMID: 18481987 Review.
Cited by
-
Identification of Bcl2 as a Stably Expressed qPCR Reference Gene for Human Colon Cancer Cells Treated with Cottonseed-Derived Gossypol and Bioactive Extracts and Bacteria-Derived Lipopolysaccharides.Molecules. 2022 Nov 4;27(21):7560. doi: 10.3390/molecules27217560. Molecules. 2022. PMID: 36364387 Free PMC article.
-
Cottonseed extracts regulate gene expression in human colon cancer cells.Sci Rep. 2022 Jan 20;12(1):1039. doi: 10.1038/s41598-022-05030-3. Sci Rep. 2022. PMID: 35058516 Free PMC article.
-
Regulation of cytoplasmic mRNA decay.Nat Rev Genet. 2012 Mar 6;13(4):246-59. doi: 10.1038/nrg3160. Nat Rev Genet. 2012. PMID: 22392217 Free PMC article. Review.
-
Stress granules in colorectal cancer: Current knowledge and potential therapeutic applications.World J Gastroenterol. 2020 Sep 21;26(35):5223-5247. doi: 10.3748/wjg.v26.i35.5223. World J Gastroenterol. 2020. PMID: 32994684 Free PMC article. Review.
-
AU-rich element-binding proteins in colorectal cancer.World J Gastrointest Oncol. 2019 Feb 15;11(2):71-90. doi: 10.4251/wjgo.v11.i2.71. World J Gastrointest Oncol. 2019. PMID: 30788036 Free PMC article. Review.
References
-
- Ciais D., Cherradi N., Bailly S., Grenier E., Berra E., Pouyssegur J., Lamarre J., Feige J. J. (2004) Oncogene 23, 8673–8680 - PubMed
-
- Suswam E., Li Y., Zhang X., Gillespie G. Y., Li X., Shacka J. J., Lu L., Zheng L., King P. H. (2008) Cancer Res. 68, 674–682 - PubMed
-
- Varnum B. C., Lim R. W., Sukhatme V. P., Herschman H. R. (1989) Oncogene 4, 119–120 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

