Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy
- PMID: 21499132
- PMCID: PMC3766324
- DOI: 10.1097/CJI.0b013e318215e300
Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy
Abstract
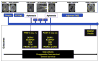
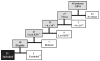
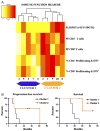
Patients with glioblastoma multiforme (GBM) are profoundly immunosuppressed and may benefit from restoration of an antitumor immune response in combination with conventional radiation therapy and temozolomide (TMZ). The optimal strategies to evaluate clinically relevant immune responses to treatment have yet to be determined. The primary objective of our study was to determine immunologic response to cervical intranodal vaccination with autologous tumor lysate-loaded dendritic cells (DCs) in patients with GBM after radiation therapy and TMZ. We used a novel hierarchical clustering analysis of immune parameters measured before and after vaccination. Secondary objectives were to assess treatment feasibility and to correlate immune response with progression-free survival (PFS) and overall survival. Ten eligible patients received vaccination. Tumor-specific cytotoxic T-cell response measured after vaccination was enhanced for the precursor frequency of CD4+ T and CD4+ interferon γ-producing cells. Hierarchical clustering analysis of multiple functional outcomes discerned 2 groups of patients according to their immune response, and additionally showed that patients in the top quintile for at least one immune function parameter had improved survival. There were no serious adverse events related to DC vaccination. All patients were alive at 6 months after diagnosis and the 6-month PFS was 90%. The median PFS was 9.5 months and overall survival was 28 months. In patients with GBM, immune therapy with DC vaccination after radiation and TMZ resulted in tumor-specific immune responses that were associated with prolonged survival. Our data suggest that DC vaccination in combination with radiation and chemotherapy in patients with GBM is feasible, safe, and may induce tumor-specific immune responses.
Conflict of interest statement
All authors have declared there are no financial conflicts of interest in regard to this work.
Figures
Similar articles
-
Phase I/IIa trial of fractionated radiotherapy, temozolomide, and autologous formalin-fixed tumor vaccine for newly diagnosed glioblastoma.J Neurosurg. 2014 Sep;121(3):543-53. doi: 10.3171/2014.5.JNS132392. Epub 2014 Jul 4. J Neurosurg. 2014. PMID: 24995786 Clinical Trial.
-
Dendritic cell vaccination combined with temozolomide retreatment: results of a phase I trial in patients with recurrent glioblastoma multiforme.J Neurooncol. 2015 Jan;121(2):319-29. doi: 10.1007/s11060-014-1635-7. Epub 2014 Oct 31. J Neurooncol. 2015. PMID: 25366363 Clinical Trial.
-
Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination.Clin Cancer Res. 2017 Apr 15;23(8):1898-1909. doi: 10.1158/1078-0432.CCR-16-2057. Clin Cancer Res. 2017. PMID: 28411277 Free PMC article. Clinical Trial.
-
Cellular-based immunotherapies for patients with glioblastoma multiforme.Clin Dev Immunol. 2012;2012:764213. doi: 10.1155/2012/764213. Epub 2012 Feb 28. Clin Dev Immunol. 2012. PMID: 22474481 Free PMC article. Review.
-
The evolution of the EGFRvIII (rindopepimut) immunotherapy for glioblastoma multiforme patients.Hum Vaccin Immunother. 2014;10(11):3322-31. doi: 10.4161/21645515.2014.983002. Hum Vaccin Immunother. 2014. PMID: 25625931 Free PMC article. Review.
Cited by
-
The future of high-grade glioma: Where we are and where are we going.Surg Neurol Int. 2015 Feb 13;6(Suppl 1):S9-S44. doi: 10.4103/2152-7806.151331. eCollection 2015. Surg Neurol Int. 2015. PMID: 25722939 Free PMC article.
-
Immunotherapy in glioblastoma: emerging options in precision medicine.CNS Oncol. 2016 Jul;5(3):175-86. doi: 10.2217/cns-2016-0009. Epub 2016 May 26. CNS Oncol. 2016. PMID: 27225028 Free PMC article. Review.
-
An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma.Expert Rev Vaccines. 2013 Jun;12(6):597-615. doi: 10.1586/erv.13.41. Expert Rev Vaccines. 2013. PMID: 23750791 Free PMC article. Review.
-
The development of dendritic cell vaccine-based immunotherapies for glioblastoma.Semin Immunopathol. 2017 Feb;39(2):225-239. doi: 10.1007/s00281-016-0616-7. Epub 2017 Jan 30. Semin Immunopathol. 2017. PMID: 28138787 Review.
-
Immune Escape in Glioblastoma Multiforme and the Adaptation of Immunotherapies for Treatment.Front Immunol. 2020 Oct 15;11:582106. doi: 10.3389/fimmu.2020.582106. eCollection 2020. Front Immunol. 2020. PMID: 33178210 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Weller M, Fontana A. The failure of current immunotherapy for malignant glioma. Tumor-derived. Brain Res Brain Res Rev. 1995;21:128–151. - PubMed
-
- Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. - PubMed
-
- Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

